Vapor Liquid Equilibrium Data for an Ideal Binary Mixture

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
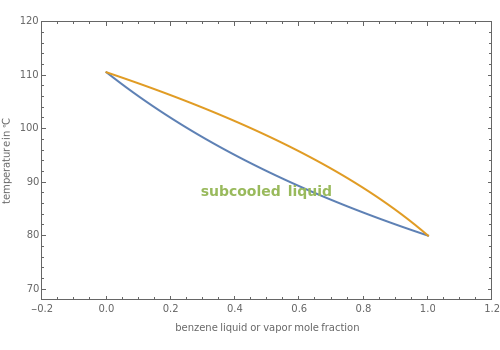
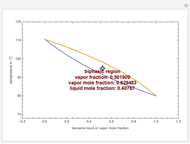
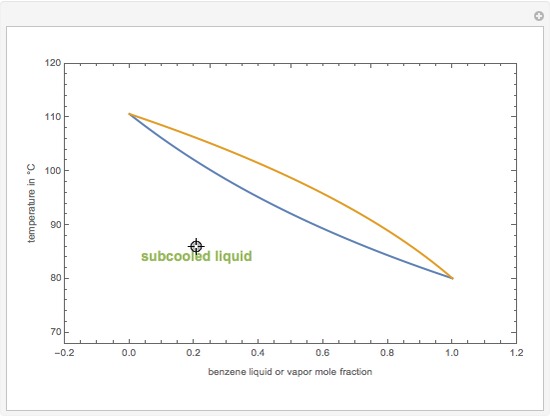
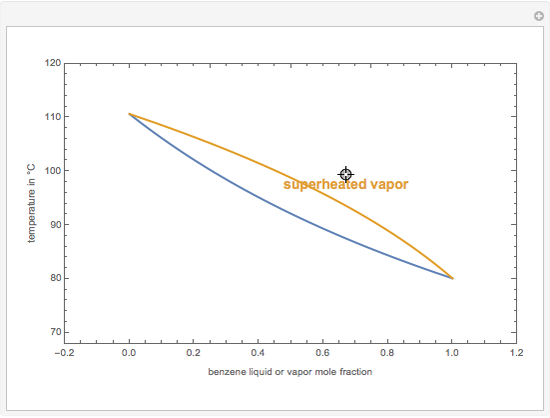
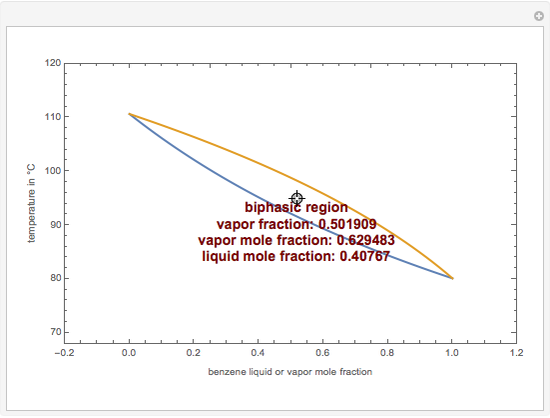
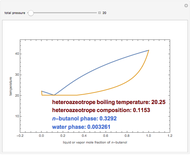
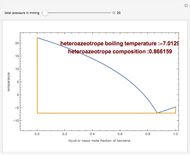
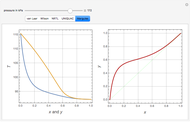
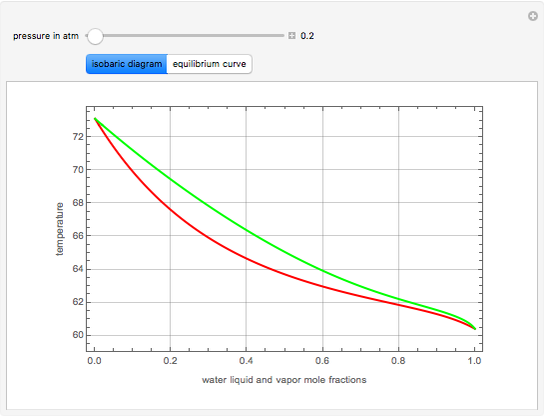
This Demonstration computes the isobaric vapor liquid equilibrium diagram at 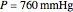 for an ideal binary mixture composed of benzene and toluene. The user can drag the locator to see the various regions delimited by the bubble and dew curves (i.e., the subcooled liquid region, the saturated liquid and vapor curves, the biphasic region, and the superheated vapor region). If the locator is in the biphasic region, the vapor fraction is calculated using the lever rule and displayed in red.
for an ideal binary mixture composed of benzene and toluene. The user can drag the locator to see the various regions delimited by the bubble and dew curves (i.e., the subcooled liquid region, the saturated liquid and vapor curves, the biphasic region, and the superheated vapor region). If the locator is in the biphasic region, the vapor fraction is calculated using the lever rule and displayed in red.
Contributed by: Housam Binous (March 2011)
Open content licensed under CC BY-NC-SA
Snapshots
Details
detailSectionParagraphPermanent Citation
"Vapor Liquid Equilibrium Data for an Ideal Binary Mixture"
http://demonstrations.wolfram.com/VaporLiquidEquilibriumDataForAnIdealBinaryMixture/
Wolfram Demonstrations Project
Published: March 7 2011