Isobaric Vapor-Liquid Equilibrium Data of an Immiscible Mixture

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
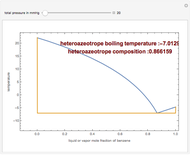
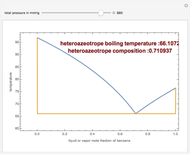
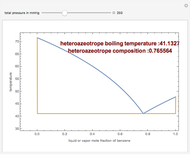
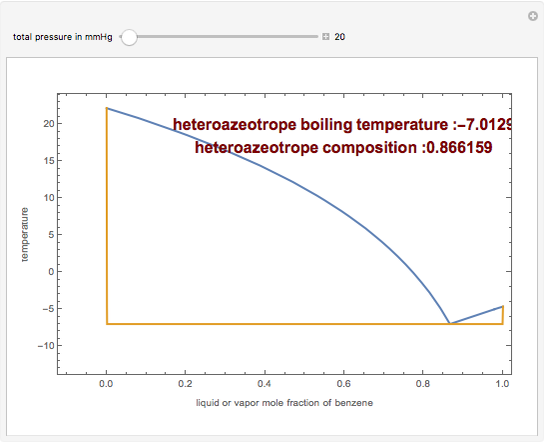
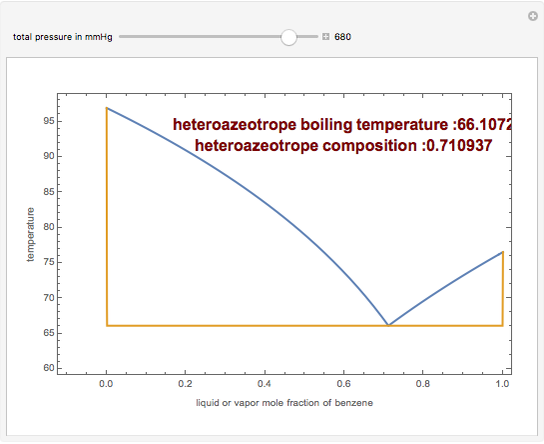
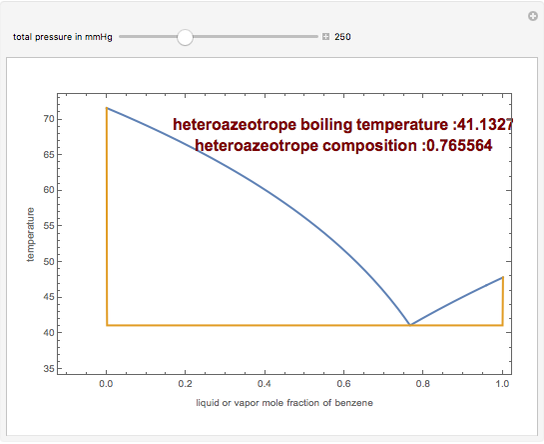
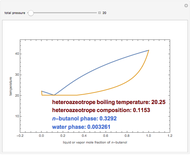
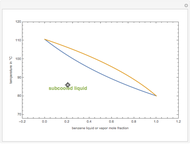
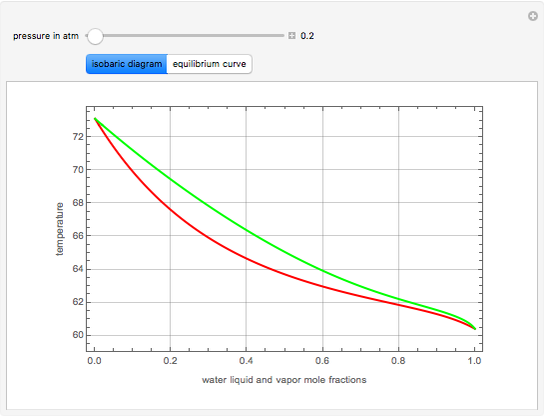
The isobaric vapor-liquid equilibrium (VLE) diagram of the immiscible binary mixture composed of benzene and water presents a heteroazeotrope. This Demonstration computes VLE data and displays the isobaric VLE diagram as well as the boiling temperature and composition of the heteroazeotrope for values of the total pressure to be set by the user. The NRTL (Non-Random Two Liquid) model, derived by Renon and Prausnitz, is used to compute the activity coefficients in the liquid phase, which are used in the modified Raoult's law: 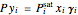 where
where  or 2,
or 2, 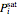 is the saturated vapor pressure of component
is the saturated vapor pressure of component 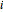 ,
, 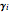 is the activity coefficient,
is the activity coefficient, 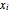 and
and 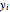 are the liquid and vapor mole fractions of component
are the liquid and vapor mole fractions of component 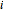 and
and 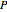 is the total pressure.
is the total pressure.
Contributed by: Housam Binous (March 2011)
Open content licensed under CC BY-NC-SA