Anatomy of a Quantum Jump

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
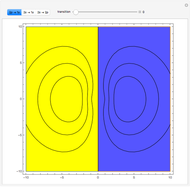
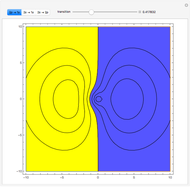
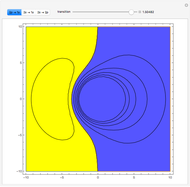
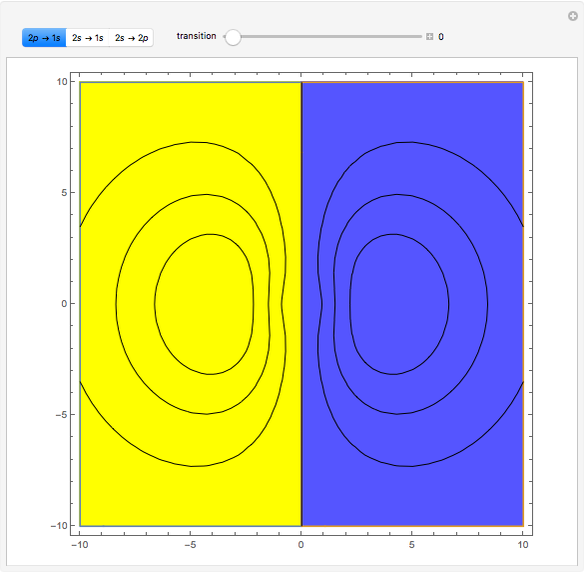
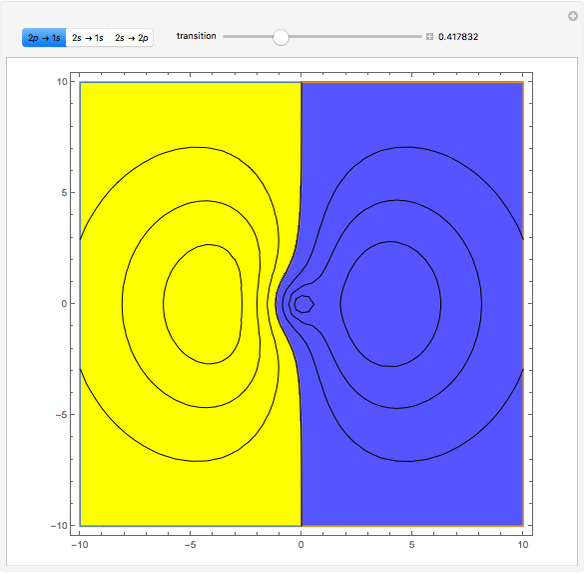
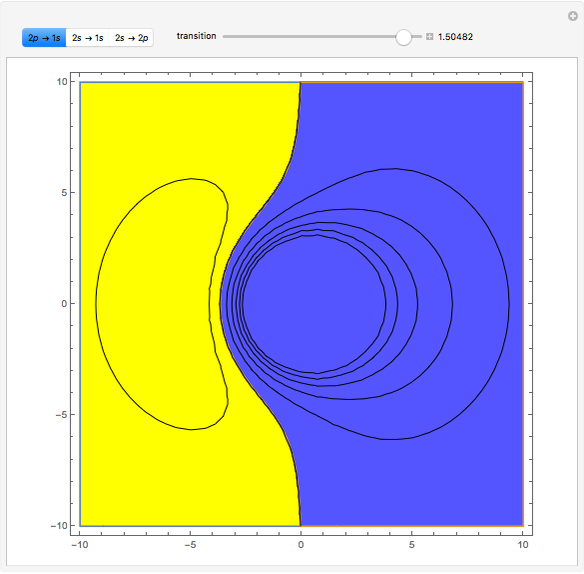
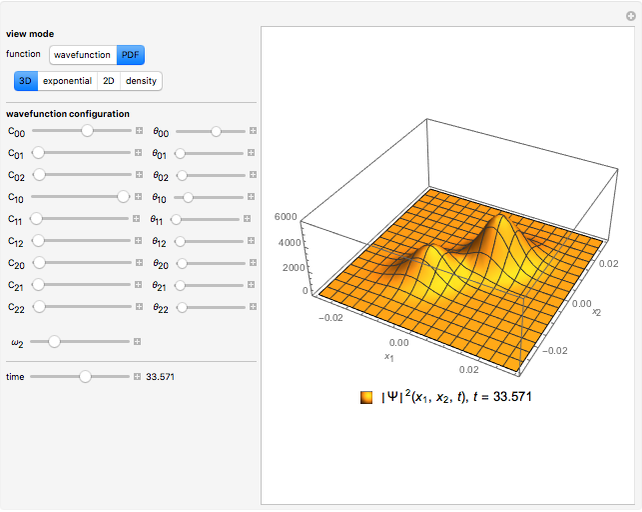
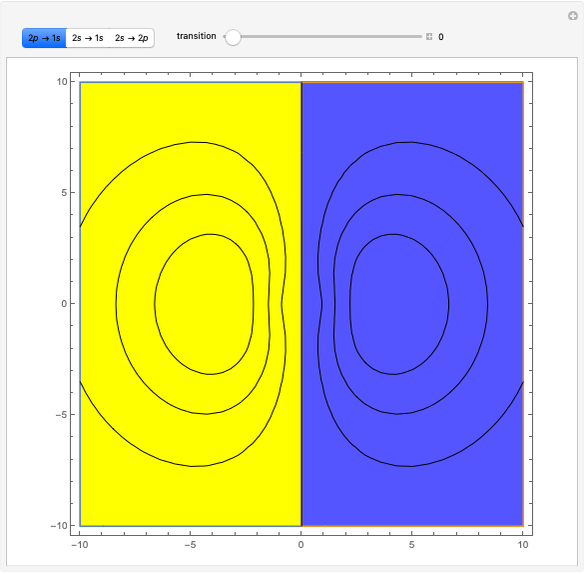
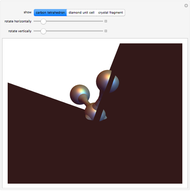
Ever since Bohr's original introduction of the quantum theory, the precise nature of transitions between discrete energy levels, with the concomitant absorption or emission of photons, has been a matter of continuing controversy. The deep philosophical issues arising from this question have been discussed in great detail by Schrödinger [1] and many others. A related phenomenon is the so-called collapse of the wavefunction. To greatly simplify the problem, the question is whether the transitions, known as quantum jumps, are truly instantaneous. Current thinking appears to be tending to the view that, to satisfy the requirements of a rational physical theory, such transitions must actually occur within a finite, although extremely short, time interval. We have conjectured that this interval might be approximated by the time it takes a light signal to traverse a Bohr radius:  sec.
sec.
Contributed by: S. M. Blinder (December 2019)
Open content licensed under CC BY-NC-SA
Details
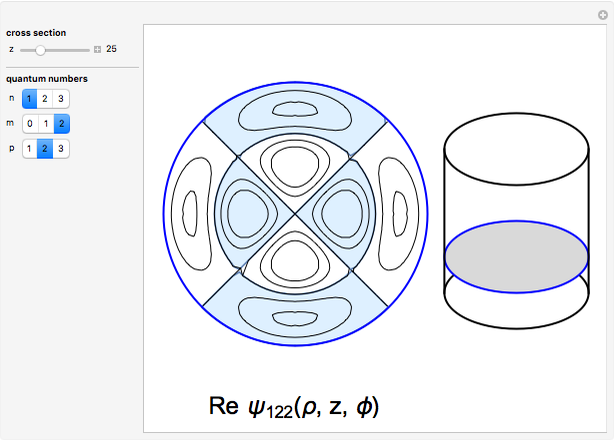
The hydrogen wavefunctions, in atomic units:
 ,
,
 ,
,
 .
.
Reference
[1] E. Schrödinger, "Are There Quantum Jumps?," The British Journal for the Philosophy of Science, 3(10) and 3(11), 1952 pp. 109–123 and 233–242. doi:10.1093/bjps/III.10.109 and doi:10.1093/bjps/III.11.233.
Snapshots
Permanent Citation