Chirality of Substituted Methanes

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
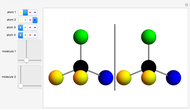
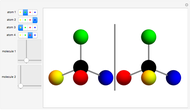
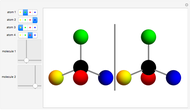
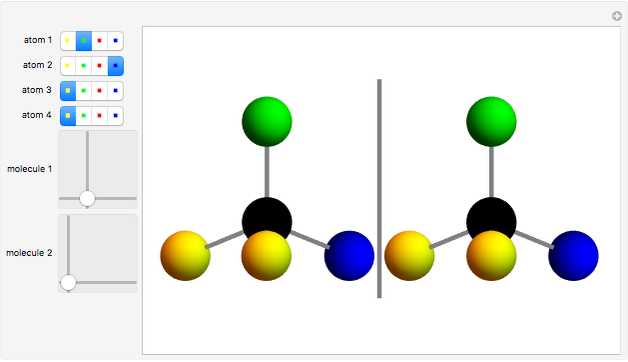
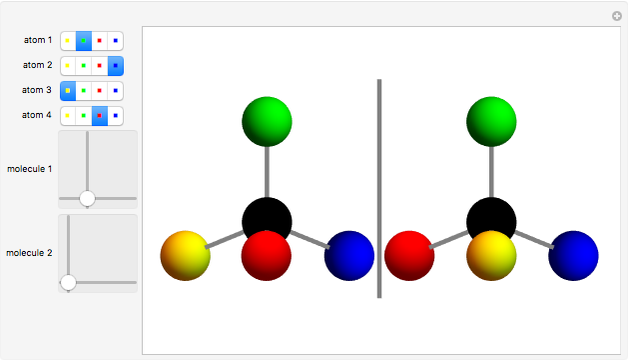
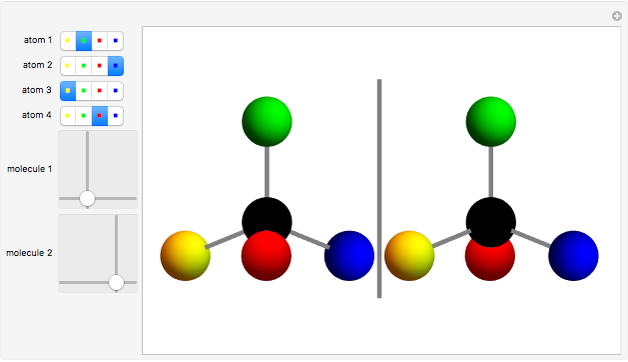
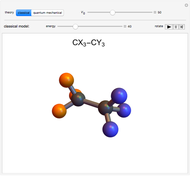
You can replace any of the H atoms in the tetrahedral methane molecule CH by other atoms, such as F, Cl or Br. When the central C is surrounded by one, two, or three different kinds of atoms, the molecule could still be superimposed on its mirror image by some manipulation of its orientation, as can be shown by using the 2D sliders. But with four different substituents bonded to the central C atom, there are distinct left- and right-handed enantiomers that possess no plane of symmetry. Such a molecule is chiral and cannot be superimposed on its mirror image. Such compounds can exhibit optical activity.
by other atoms, such as F, Cl or Br. When the central C is surrounded by one, two, or three different kinds of atoms, the molecule could still be superimposed on its mirror image by some manipulation of its orientation, as can be shown by using the 2D sliders. But with four different substituents bonded to the central C atom, there are distinct left- and right-handed enantiomers that possess no plane of symmetry. Such a molecule is chiral and cannot be superimposed on its mirror image. Such compounds can exhibit optical activity.
Contributed by: S. M. Blinder (March 2011)
Open content licensed under CC BY-NC-SA
Snapshots
Details
Snapshot 1: By the appropriate rotations, the two molecules shown could be superimposed.
Snapshot 2: With four different substituents, the mirror image molecules can never be superimposed.
Snapshot 3: This heroic attempt fails!
Permanent Citation
"Chirality of Substituted Methanes"
http://demonstrations.wolfram.com/ChiralityOfSubstitutedMethanes/
Wolfram Demonstrations Project
Published: March 7 2011