Fluids in the Critical Region

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
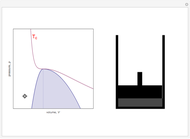
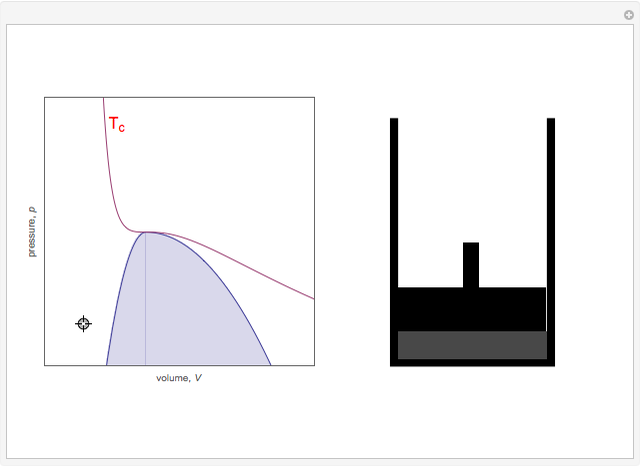
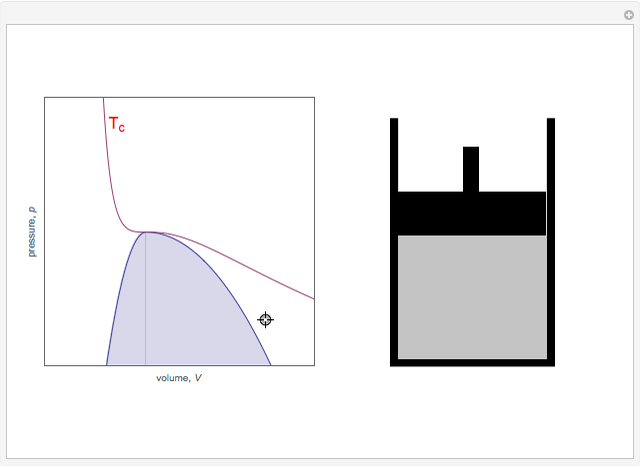
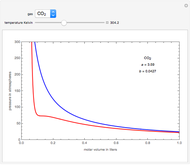
On the left is a phase diagram of the critical region of a representative fluid. Within the shaded area, the fluid separates into two phases: liquid and gas. The critical isotherm is also shown, labeled by the critical temperature  . At the critical point, this isotherm is tangent to the two-phase region. A gas can be liquefied only after it is cooled below its critical temperature.
. At the critical point, this isotherm is tangent to the two-phase region. A gas can be liquefied only after it is cooled below its critical temperature.
Contributed by: S. M. Blinder (March 2011)
Open content licensed under CC BY-NC-SA
Snapshots
Details
Snapshot 1: This state would be identified as a liquid at a given pressure.
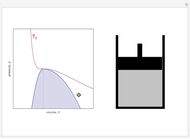
Snapshot 2: At a much higher temperature, the system would be a gas at the same pressure.
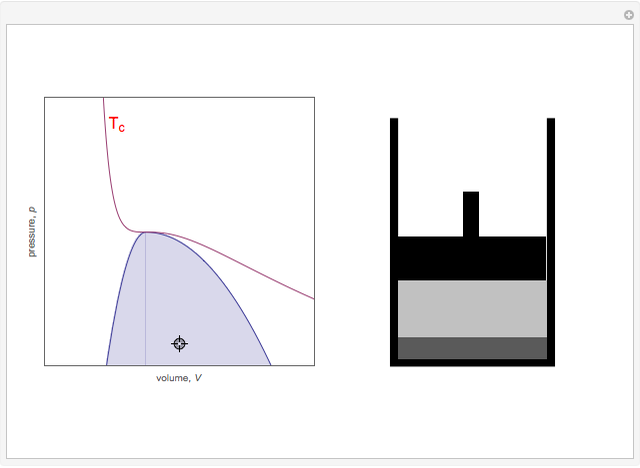
Snapshot 3: Going from state 1 to state 2 at constant pressure, the system would go through the two-phase region.
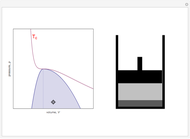
Snapshot 4: An alternative way to go from state 1 to state 2 would be to follow a path around the critical point. The same final state would then be attained continuously, without any phase separation. This is known as the principle of continuity of states.
Snapshot 5: As the critical point is approached, the densities of the two phases approach one another.
Snapshot 6: The densities become equal at the critical point.
Reference: S. M Blinder, Advanced Physical Chemistry; A Survey of Modern Theoretical Principles, New York: Macmillan, 1969 p. 134.
Permanent Citation
"Fluids in the Critical Region"
http://demonstrations.wolfram.com/FluidsInTheCriticalRegion/
Wolfram Demonstrations Project
Published: March 7 2011