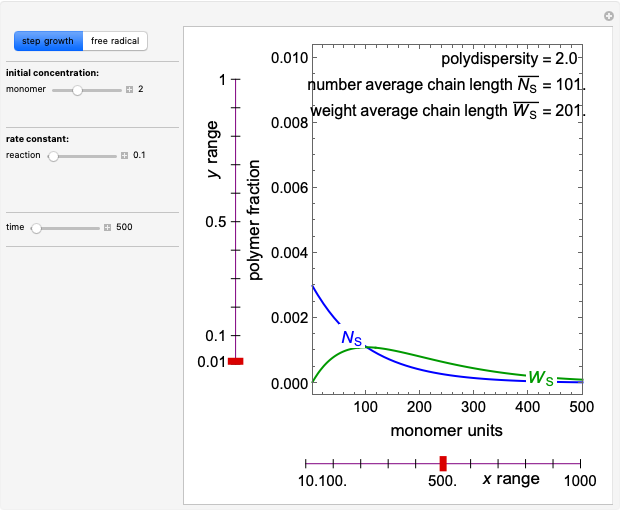
Mass Balances in Evaporative Crystallization

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
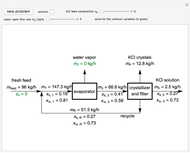
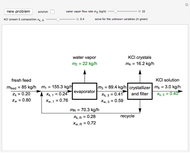
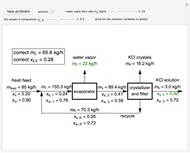
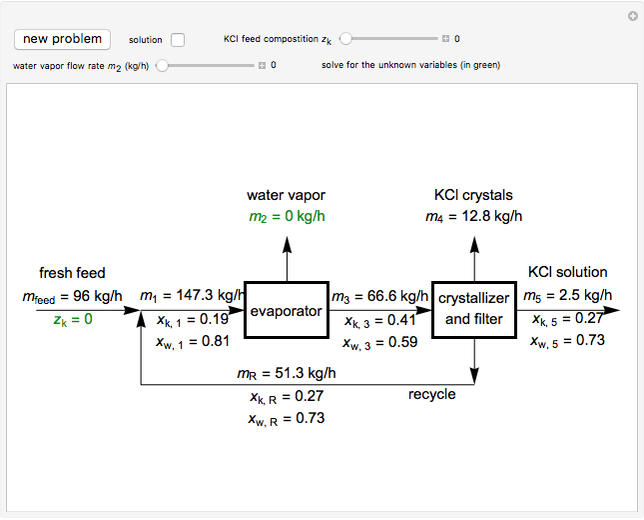
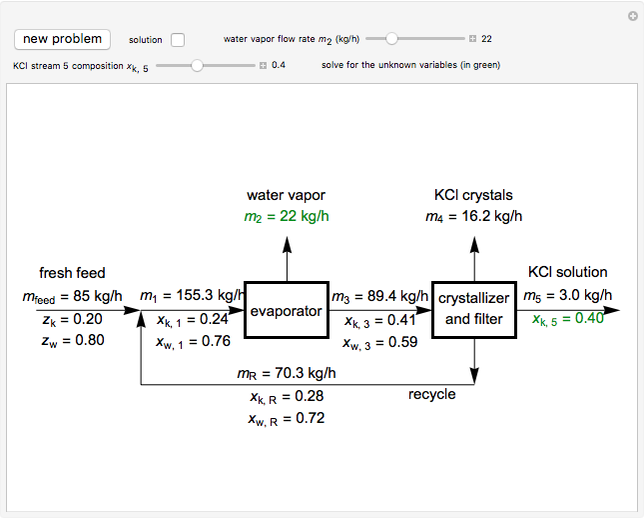
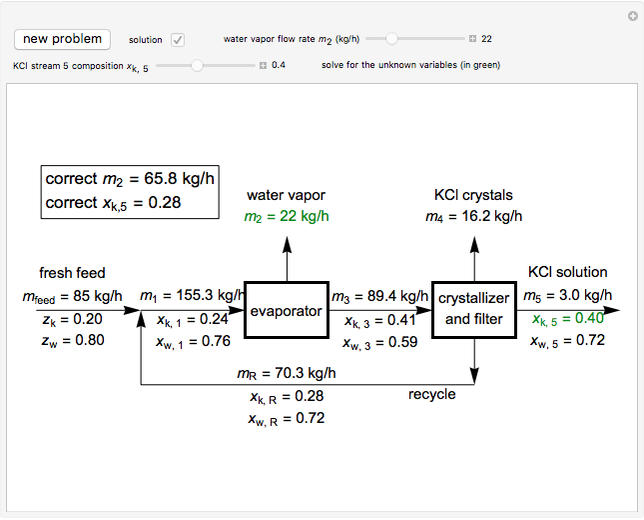
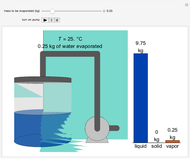
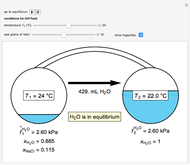
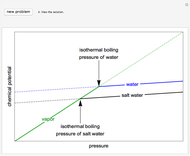
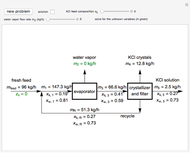
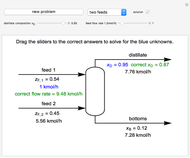
In this Demonstration, you can perform mass balances on an evaporative crystallization process. Use the sliders to select the correct solution for the variables shown in green, then check the "solution" box to verify your answers. Click the "new problem" button for a new set of conditions.
Contributed by: Rachael L. Baumann and Neil Hendren (November 2018)
Additional contributions by: John L. Falconer
(University of Colorado Boulder, Department of Chemical and Biological Engineering)
Open content licensed under CC BY-NC-SA
Details
Overall material balances
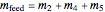
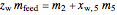
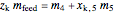
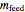 = mass flow rate of fresh feed (kg/h)
= mass flow rate of fresh feed (kg/h)
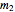 = mass flow rate of water exiting evaporator (kg/h)
= mass flow rate of water exiting evaporator (kg/h)
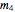 = mass flow rate of solid KCl crystals exiting the crystallizer and filter (kg/h)
= mass flow rate of solid KCl crystals exiting the crystallizer and filter (kg/h)
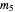 = mass flow rate of slurry exiting the crystallizer and filter (kg/h)
= mass flow rate of slurry exiting the crystallizer and filter (kg/h)
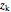 = mass fraction of KCl in fresh feed
= mass fraction of KCl in fresh feed
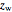 = mass fraction of water in fresh feed
= mass fraction of water in fresh feed
Material balance around crystallizer and filter

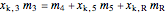
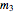 = mass flow rate of solution exiting evaporator (kg/h)
= mass flow rate of solution exiting evaporator (kg/h)
 = mass flow rate of recycle (kg/h)
= mass flow rate of recycle (kg/h)
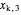 = mass fraction of KCl in stream exiting evaporator
= mass fraction of KCl in stream exiting evaporator
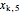 = mass fraction of KCl in solution exiting crystallizer and filter
= mass fraction of KCl in solution exiting crystallizer and filter
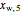 = mass fraction of water in solution exiting crystallizer and filter (this is equivalent to
= mass fraction of water in solution exiting crystallizer and filter (this is equivalent to 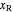 as the exit stream from the crystallizer is split)
as the exit stream from the crystallizer is split)
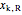 = mass fraction of KCl in recycle stream
= mass fraction of KCl in recycle stream
Material balances around mixing point
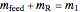
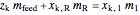
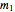 = mass flow rate entering evaporator (kg/h)
= mass flow rate entering evaporator (kg/h)
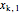 = mass fraction of KCl in stream entering evaporator
= mass fraction of KCl in stream entering evaporator
Material balance around evaporator
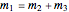
Dependence of solubility on temperature
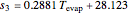
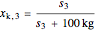
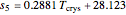

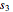 = solubility of KCl in 100 kg
= solubility of KCl in 100 kg 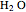
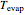 = evaporator operating temperature (°C)
= evaporator operating temperature (°C)
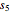 = solubility of KCl in 100 kg
= solubility of KCl in 100 kg 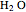
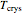 = crystallizer and filter operating temperature (°C)
= crystallizer and filter operating temperature (°C)
Note that 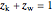 and
and 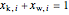 , where
, where 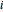 is the stream.
is the stream.
Recovery rate 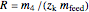
View the screencast video at [1] for more practice with material balances on a crystallizer.
Reference
[1] University of Colorado. Crystallizer Material Balance with Recycle [Video]. (Oct 29, 2013) www.youtube.com/watch?v=Dnfgc4_OFhI.
Snapshots
Permanent Citation