Reaction in an Adiabatic Continuous Stirred-Tank Reactor

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
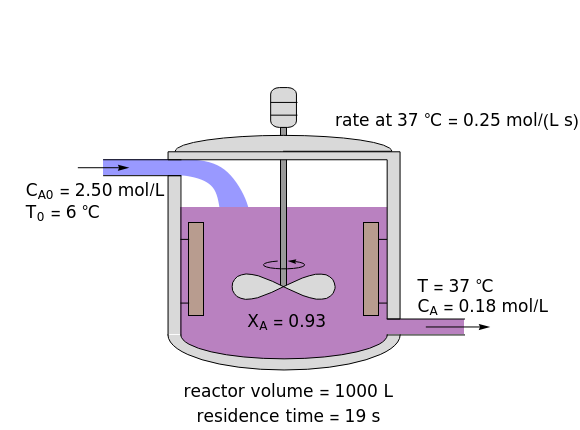
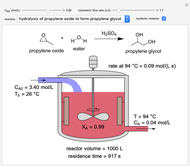
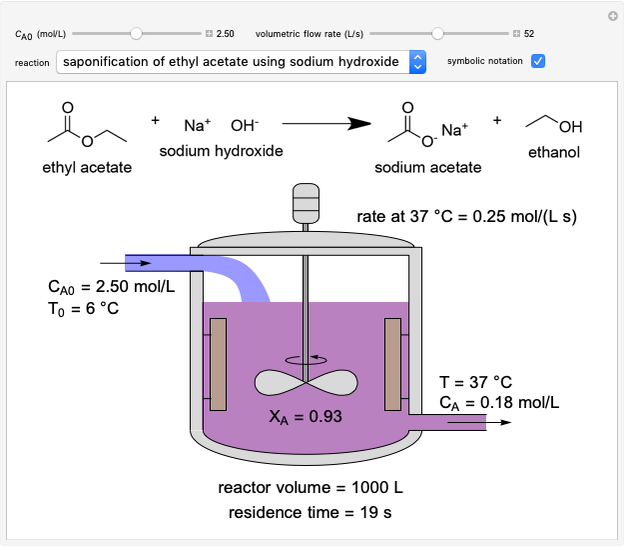
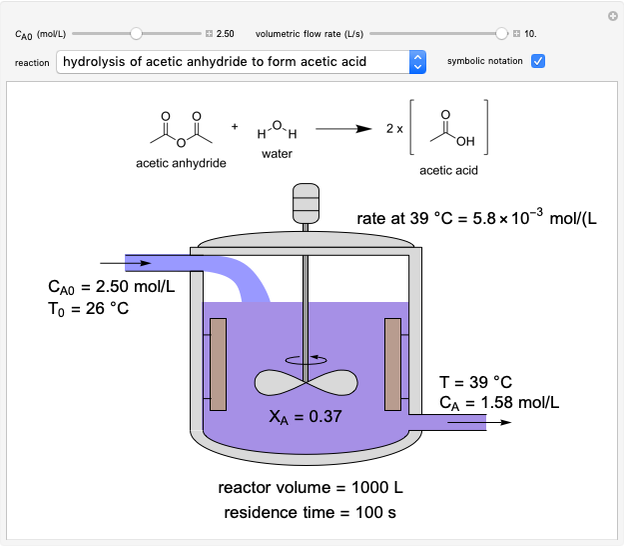
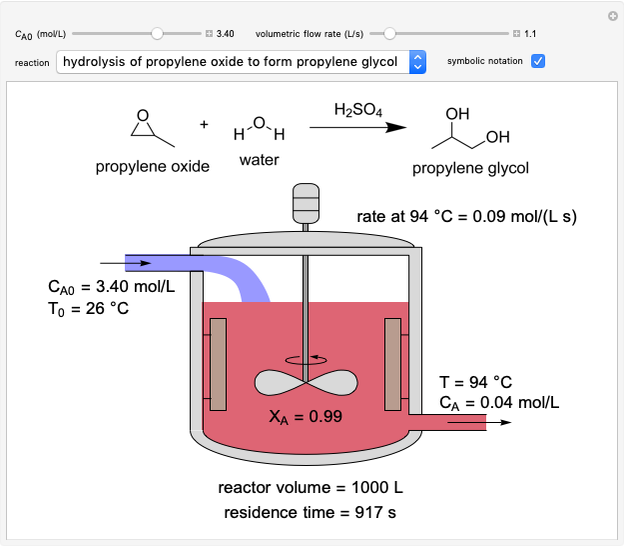
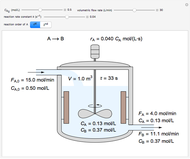
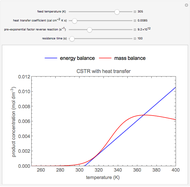
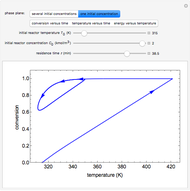
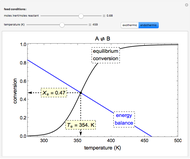
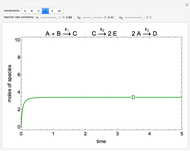
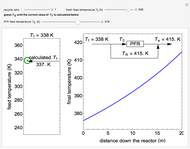
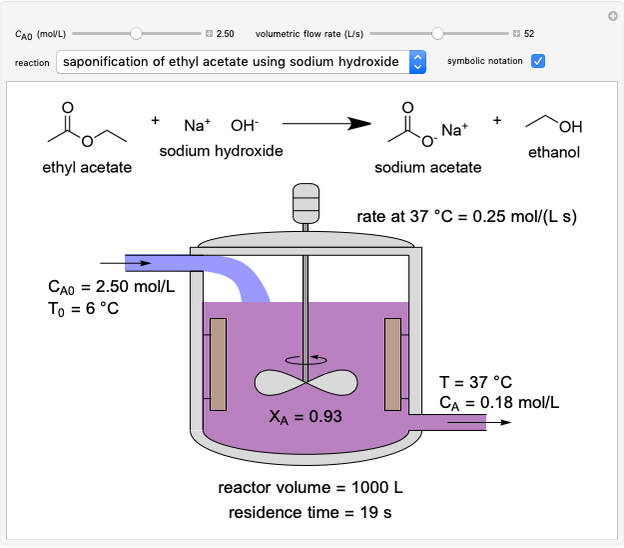
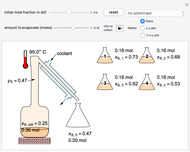
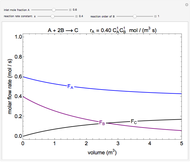
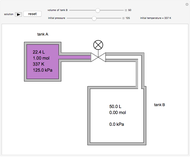
In this Demonstration, a liquid-phase reaction takes place in an adiabatic, continuous stirred-tank reactor (CSTR). Select an exothermic reaction, and then select the feed concentration of the limiting reactant  (
( ) and the volumetric flow rate. Mass and energy balances are solved to determine the outlet reactant concentration and temperature. The color of the fluid in the reactor is correlated with the reactor temperature: lower temperature is blue, and as the temperature increases, the fluid becomes purple and then red. The fractional conversion of reactant
) and the volumetric flow rate. Mass and energy balances are solved to determine the outlet reactant concentration and temperature. The color of the fluid in the reactor is correlated with the reactor temperature: lower temperature is blue, and as the temperature increases, the fluid becomes purple and then red. The fractional conversion of reactant  is represented by
is represented by  . Symbolic representation of the reaction may be toggled on and off by selecting "symbolic notation".
. Symbolic representation of the reaction may be toggled on and off by selecting "symbolic notation".
Contributed by: Neil Hendren (July 2020)
Additional contributions by: John L. Falconer
Open content licensed under CC BY-NC-SA
Snapshots
Details
At steady state, the rate of reaction is constant within the CSTR, so that the total moles reacted per time equals reaction rate times volume:

where  and
and  are the inlet and outlet molar flow rates of reactant
are the inlet and outlet molar flow rates of reactant  ,
,  is rate of reaction (mol/L s) of
is rate of reaction (mol/L s) of  and
and  is reactor volume. This equation may be expressed in terms of residence time
is reactor volume. This equation may be expressed in terms of residence time  , conversion
, conversion  and inlet concentration of
and inlet concentration of  ,
,  :
:
 .
.
The rate expression  is a function of reactant concentrations and the rate constant
is a function of reactant concentrations and the rate constant  :
:
 .
.
The rate constant  increases with temperature according to the Arrhenius equation:
increases with temperature according to the Arrhenius equation:

where  is the pre-exponential factor,
is the pre-exponential factor,  is the activation energy of the reaction,
is the activation energy of the reaction,  is the gas constant and
is the gas constant and  is absolute temperature. Energy released from exothermic reactions causes the reactor contents (and the reactor effluent) to have a higher temperature than the inlet:
is absolute temperature. Energy released from exothermic reactions causes the reactor contents (and the reactor effluent) to have a higher temperature than the inlet:

where  is the total molar flow rate at the inlet or outlet,
is the total molar flow rate at the inlet or outlet,  is the molar heat capacity of the mixture within the reactor,
is the molar heat capacity of the mixture within the reactor,  is temperature at the inlet and
is temperature at the inlet and  is the heat of reaction.
is the heat of reaction.
Permanent Citation