Simple Batch Distillation of an Ethanol-Water Mixture

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
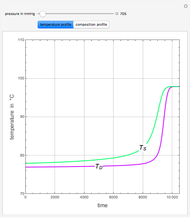
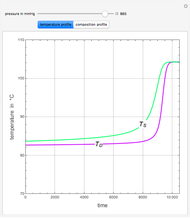
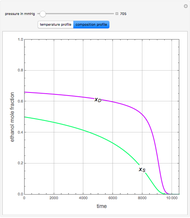
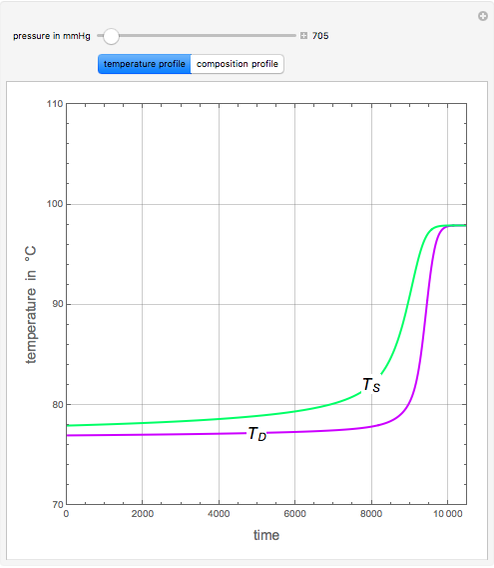
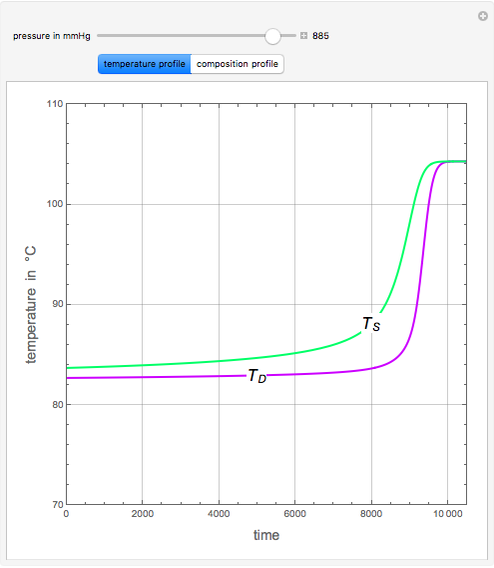
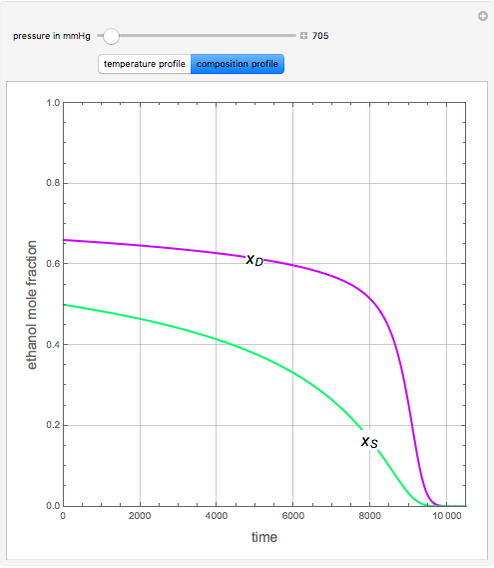
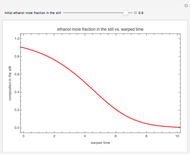
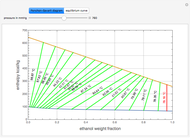
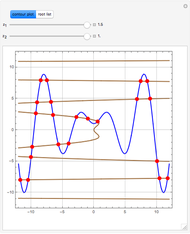
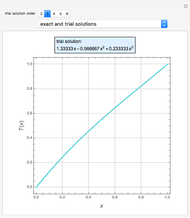
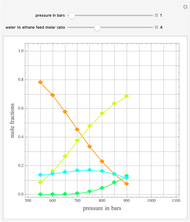
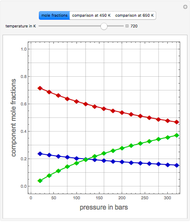
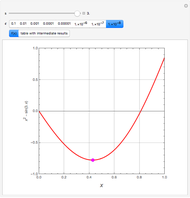
An equimolar binary mixture of ethanol and water is to be separated in a still pot, which initially contains 0.575 kmol of the mixture. You can set the operating pressure,  , of this simple batch distillation experiment, where
, of this simple batch distillation experiment, where  can vary from 700 to 900 mmHg so that the ideal gas-phase assumption holds.
can vary from 700 to 900 mmHg so that the ideal gas-phase assumption holds.
Contributed by: Housam Binous, Mamdouh Al-Harthi, and Brian G. Higgins (December 2011)
Open content licensed under CC BY-NC-SA
Snapshots
Details
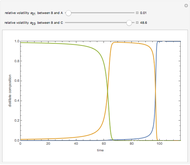
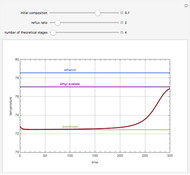
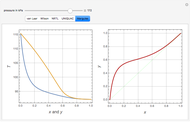
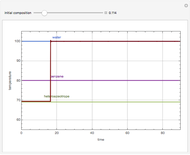
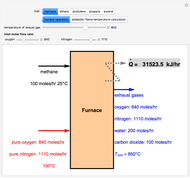
With the simple experimental apparatus shown below, one can obtain the temperature in the still pot,  , and that of the escaping vapor,
, and that of the escaping vapor,  . Since the system is a binary mixture, the composition
. Since the system is a binary mixture, the composition  in the still pot can be inferred from the temperature measurement
in the still pot can be inferred from the temperature measurement  obtained using a thermocouple with digital display.
obtained using a thermocouple with digital display.
Permanent Citation