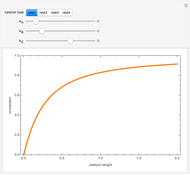
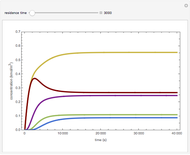
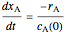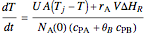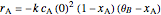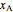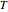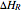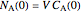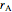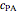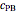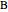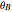Endothermic Reaction in a Heated Batch Reactor

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
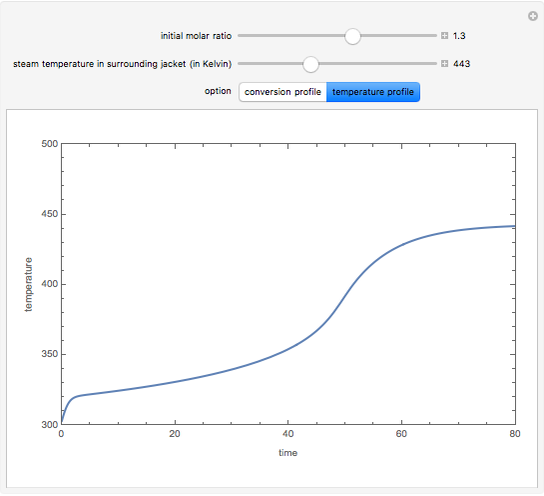
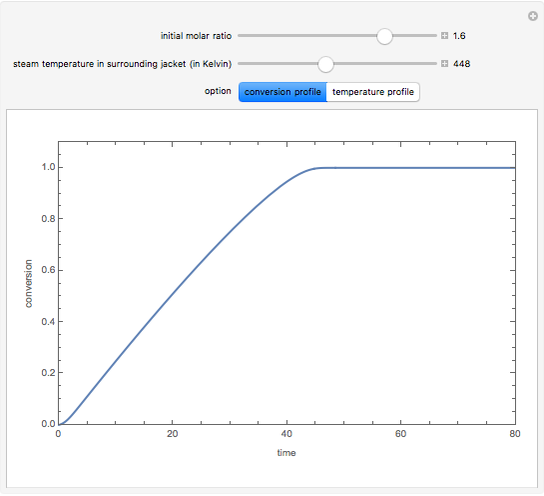
Consider an irreversible endothermic reaction (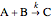 ) in a batch reactor heated by a steam jacket at temperature
) in a batch reactor heated by a steam jacket at temperature 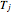 surrounding the reactor. The jacket heat transfer area and coefficient are
surrounding the reactor. The jacket heat transfer area and coefficient are 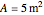 and
and 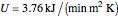 . The reactor volume is
. The reactor volume is 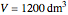 . The reaction rate constant is given by
. The reaction rate constant is given by 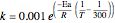 , with the activation energy
, with the activation energy 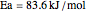 and the universal gas constant
and the universal gas constant 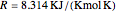 . The initial concentration of
. The initial concentration of 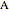 is
is 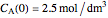 and
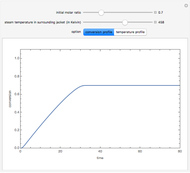
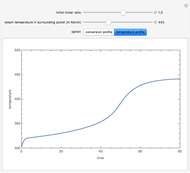
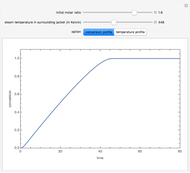
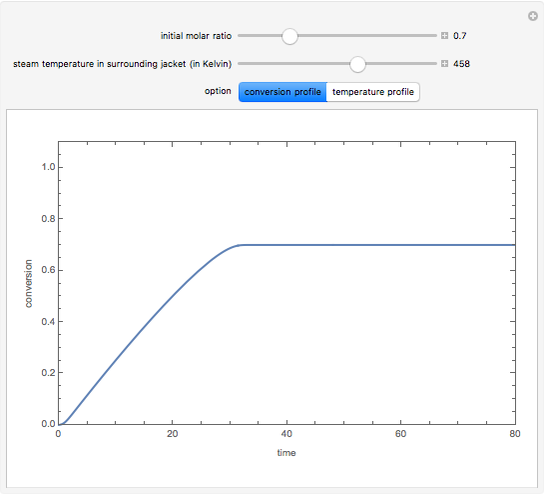
and 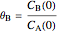 is the initial molar ratio. The initial reactor temperature is 30°C. The mass and energy balances are
is the initial molar ratio. The initial reactor temperature is 30°C. The mass and energy balances are
Contributed by: Housam Binous (November 2008)
Open content licensed under CC BY-NC-SA
Snapshots
Details
Reference
M. B. Cutlip and M. Shacham, Problem Solving in Chemical Engineering with Numerical Methods, Upper Saddle River, N.J.: Prentice Hall, 1999.
Permanent Citation