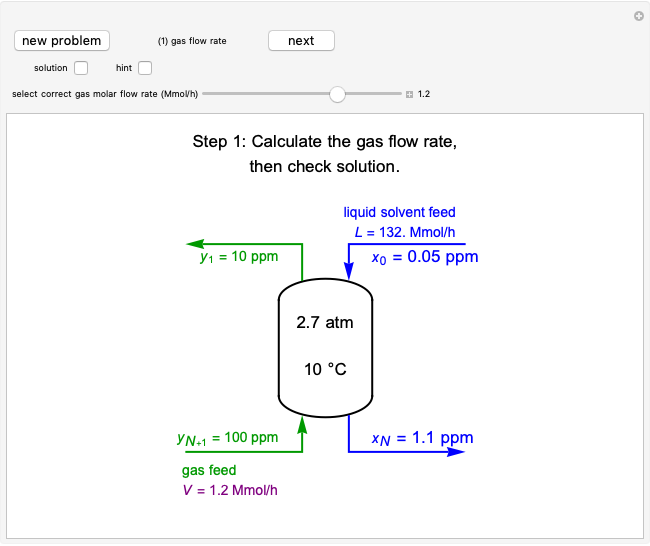
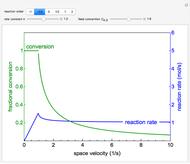
Temperature-Programmed Desorption

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
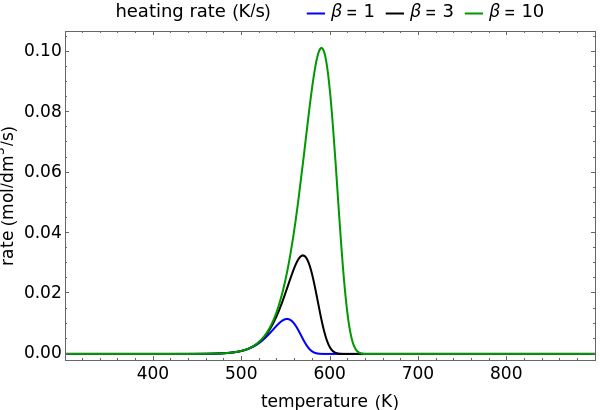
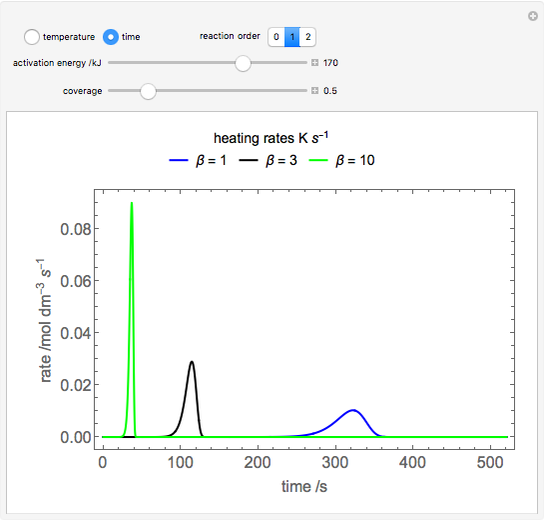
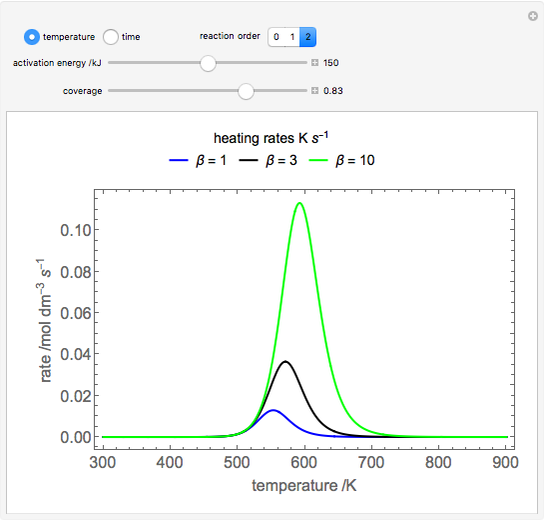
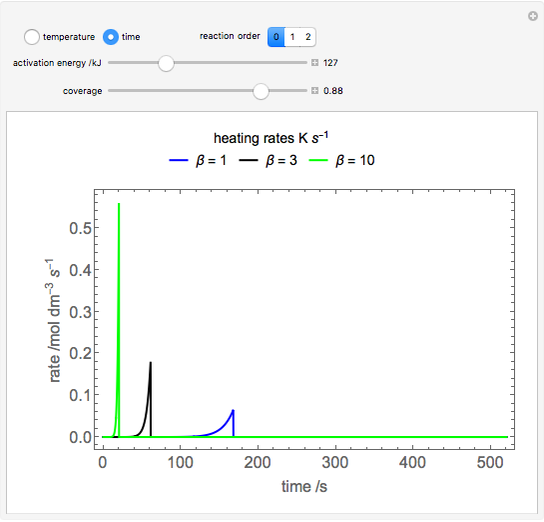
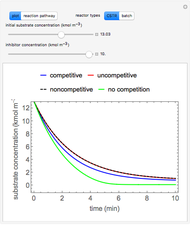
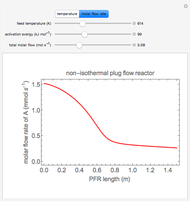
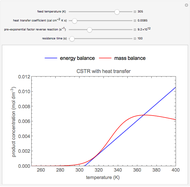
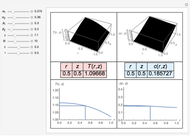
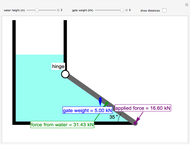
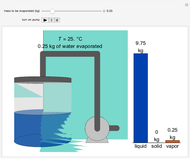
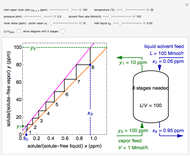
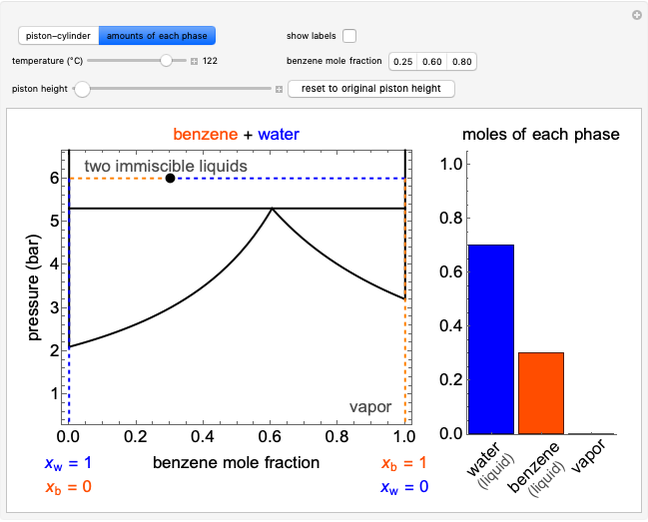
During temperature-programmed desorption (TPD), a molecule is adsorbed on a solid surface, the temperature is ramped linearly with time and the rate of desorption is measured. The rates of desorption are plotted versus either temperature or time (select with buttons) for three heating rates (1, 3 and 10 K/s). Select the desorption order with buttons; vary the desorption activation energy and the initial fractional surface coverage with sliders.
Contributed by: Rachael L. Baumann (June 2013)
Additional contributions by: John L. Falconer and Nick Bongiardina
Open content licensed under CC BY-NC-SA
Snapshots
Details
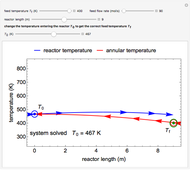
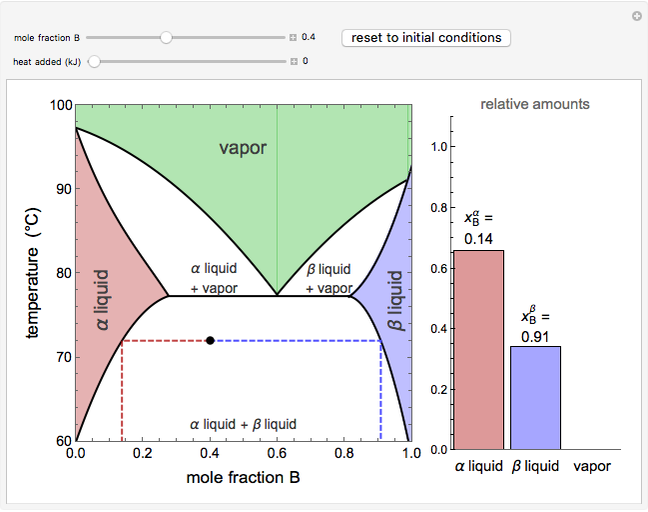
As the heating rate increases, the temperature at which the desorption rate is a maximum (peak temperature) increases, and the maximum rate of desorption increases. When plotted on a time scale, as the heating rate increases, the maximum rate of desorption occurs at shorter times. As the activation energy for desorption increases, the peak temperature increases. This is one of the most widely-used techniques for characterizing catalysts and porous materials and for studying catalytic mechanisms.
Mole balance:
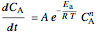 ,
,
where 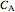 is the concentration of
is the concentration of 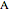 (mol/L),
(mol/L),  is time (s), at
is time (s), at  ,
, 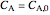 is the saturation coverage (mol/L),
is the saturation coverage (mol/L),  is order of reaction,
is order of reaction, 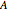 is a pre-exponential factor,
is a pre-exponential factor, 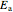 is activation energy (kJ/mol),
is activation energy (kJ/mol), 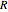 is the ideal gas constant (kJ/(mol K)) and
is the ideal gas constant (kJ/(mol K)) and 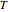 is temperature (K).
is temperature (K).
Temperature-time relation:
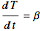 ,
,
where 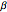 is the heating rate (K/s).
is the heating rate (K/s).
The screencast video at [1] shows how to use this Demonstration.
Reference
[1] Temperature-Programmed Desorption [Video]. (Jan 20, 2017) www.colorado.edu/learncheme/kinetics/TPD.xhtml.
Permanent Citation