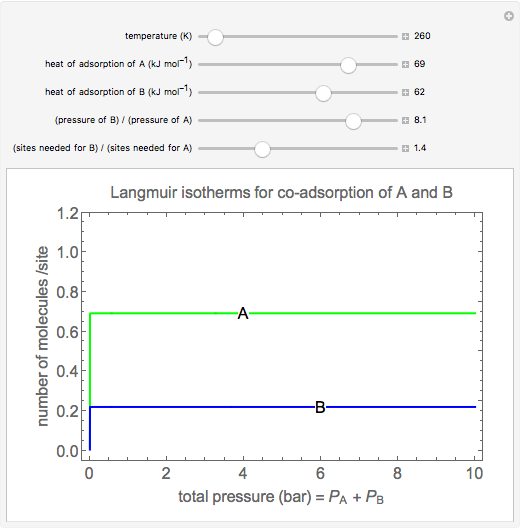
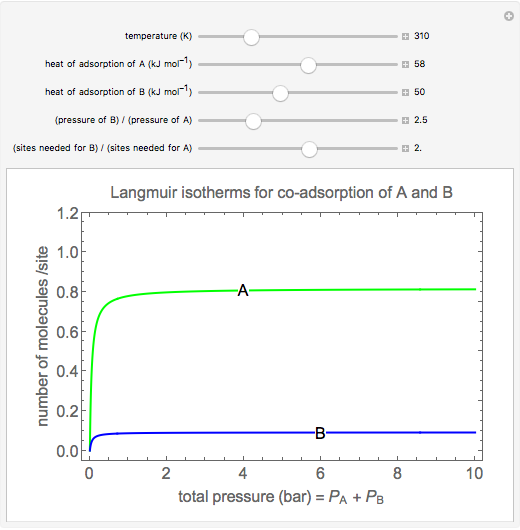
Langmuir Isotherms for a Binary Mixture

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
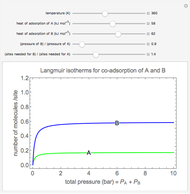
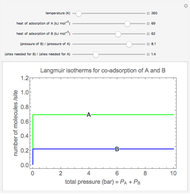
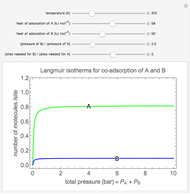
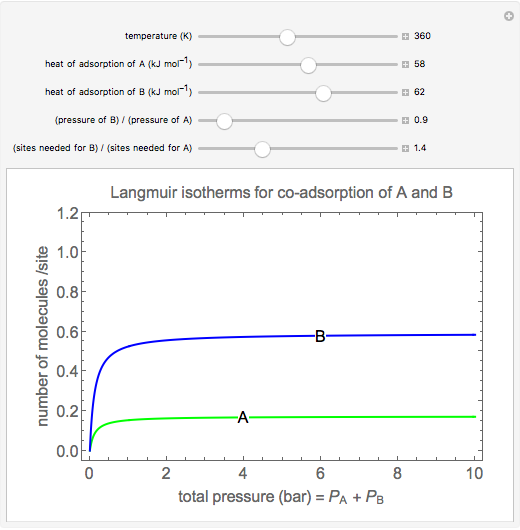
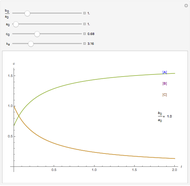
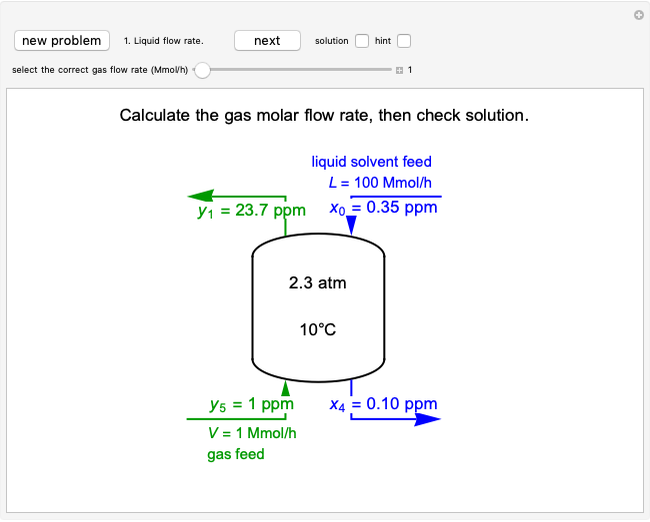
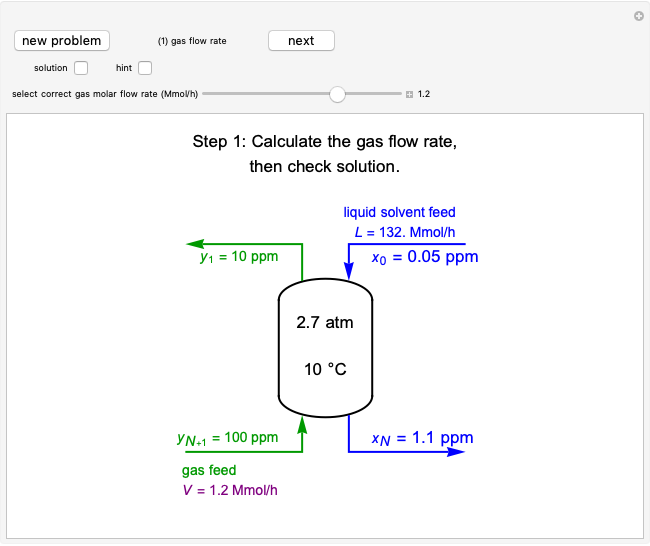
Langmuir isotherms are generated for each component in a binary gas-phase mixture of  and
and  . Vary the heats of adsorption of each component, the temperature, and the ratio of partial pressures with sliders. The molecules compete for adsorption sites but do not interact with each other, and this is taken into account in the form of the Langmuir isotherm. Vary the relative number of sites per molecule with a slider to account for larger molecules occupying more surface area than smaller molecules. The Langmuir isotherm for molecules
. Vary the heats of adsorption of each component, the temperature, and the ratio of partial pressures with sliders. The molecules compete for adsorption sites but do not interact with each other, and this is taken into account in the form of the Langmuir isotherm. Vary the relative number of sites per molecule with a slider to account for larger molecules occupying more surface area than smaller molecules. The Langmuir isotherm for molecules  and
and  has the form:
has the form:
Contributed by: Rachael L. Baumann (February 2014)
Additional contributions by: John L. Falconer and Nick Bongiardina
(University of Colorado, Department of Chemical and Biological Engineering)
Open content licensed under CC BY-NC-SA
Snapshots
Details
The number of molecules per site for components  and
and  are:
are:
 ,
,
 ,
,
where  is the adsorption equilibrium constant for component
is the adsorption equilibrium constant for component 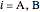 (1/bar),
(1/bar),  is the partial pressure (bar), and
is the partial pressure (bar), and  is the saturation coverage of
is the saturation coverage of  to
to  .
.
 ,
,
where  is a pre-exponential factor (1/bar),
is a pre-exponential factor (1/bar),  is the heat of adsorption (kJ/mol),
is the heat of adsorption (kJ/mol),  is the ideal gas constant (kJ/[mol K]), and
is the ideal gas constant (kJ/[mol K]), and  is temperature (K).
is temperature (K).
 ,
,
 ,
,
where  is the total pressure
is the total pressure  (bar), and
(bar), and  is the ratio of partial pressures.
is the ratio of partial pressures.
The screencast video at [1] shows how to use this Demonstration.
Reference
[1] Langmuir Isotherms for a Binary Mixture. www.colorado.edu/learncheme/kinetics/LanmuirIsothermsBinaryMixture.html.
Permanent Citation