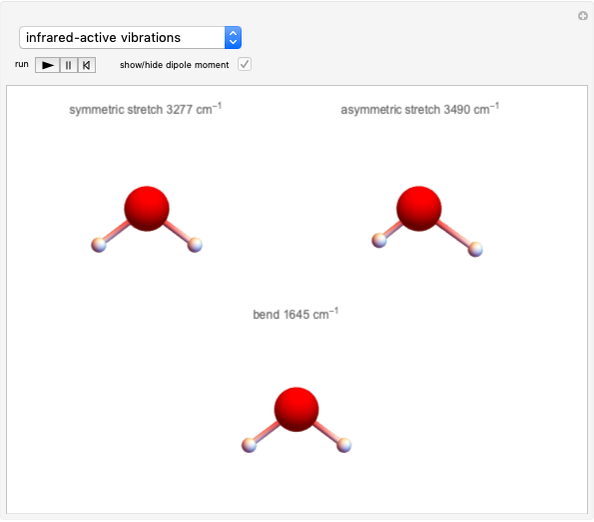
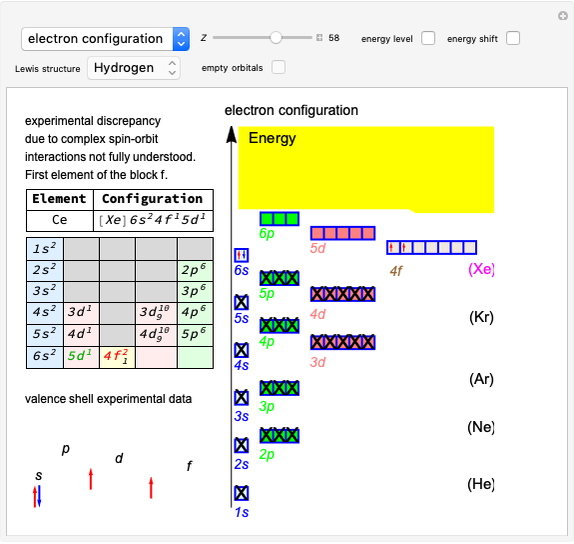
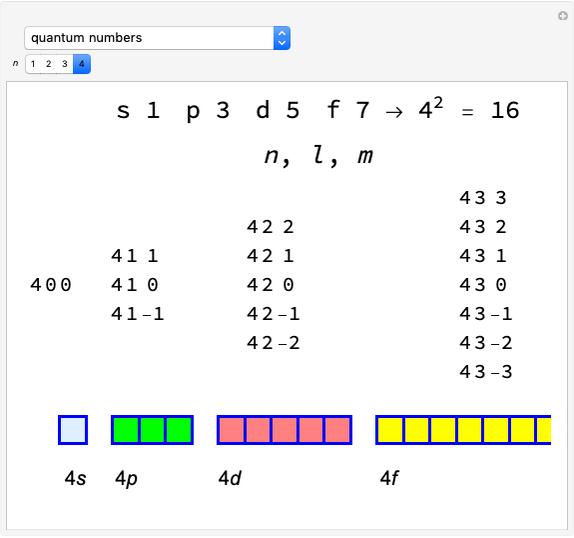
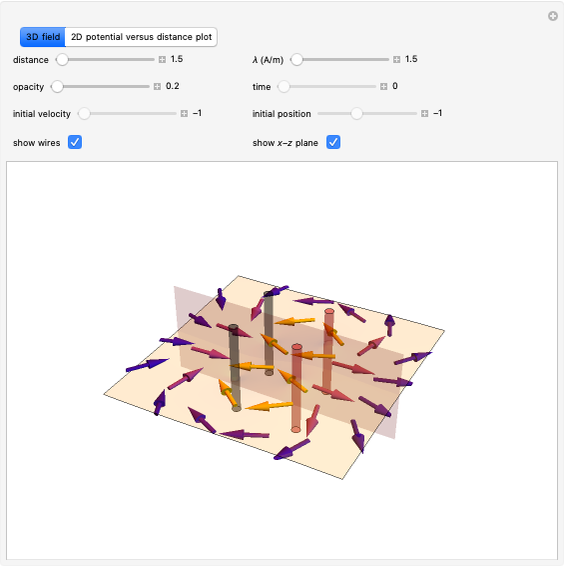
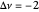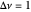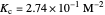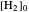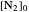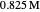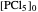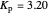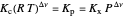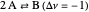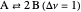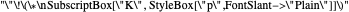Effect of Pressure on Chemical Equilibrium

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
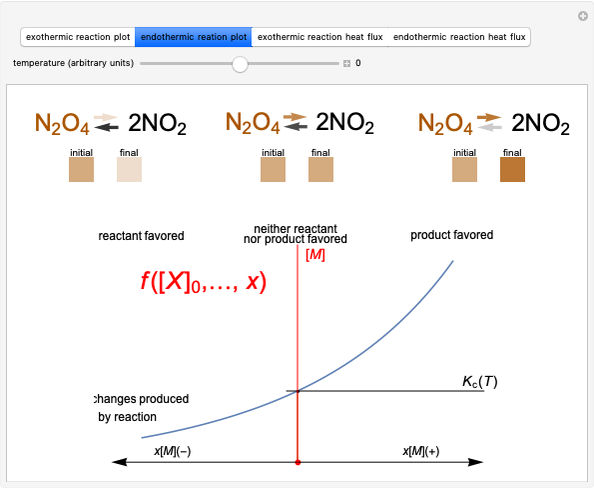
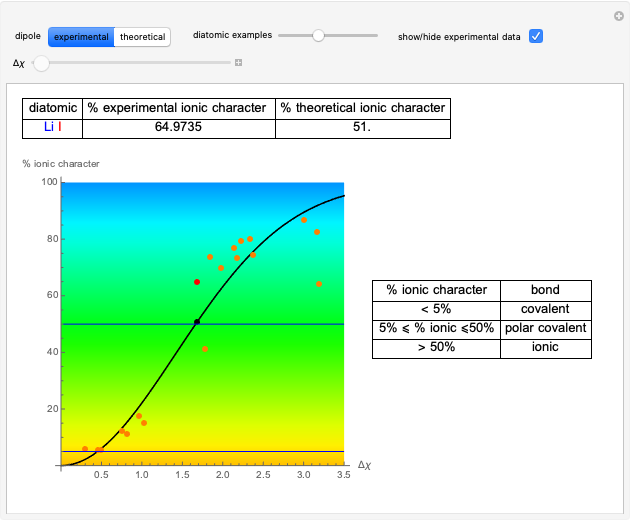
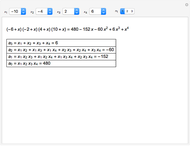
This Demonstration shows the displacement of a chemical equilibrium following pressure variation, a consequence of Le Chatelier's principle. In order for this shift to take place it is necessary that  [1], namely that the sums of the stoichiometric coefficients for the products and for the reactants are not equal.
[1], namely that the sums of the stoichiometric coefficients for the products and for the reactants are not equal.
Contributed by: D. Meliga, L. Lavagnino and S. Z. Lavagnino (December 2020)
Additional contribution by: G. Follo
Open content licensed under CC BY-NC-SA
Snapshots
Details
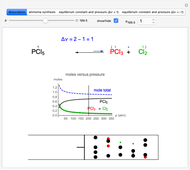
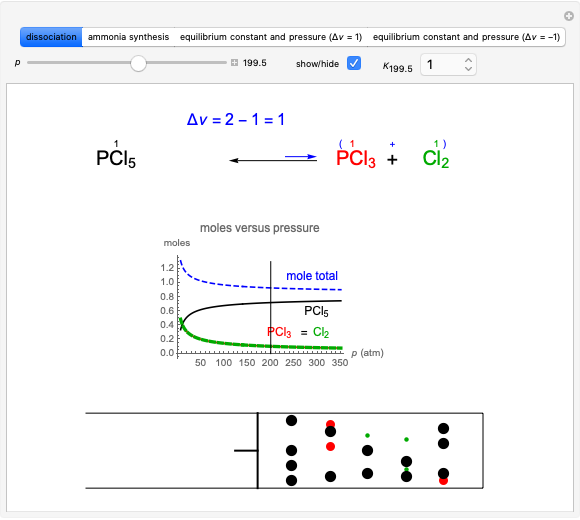
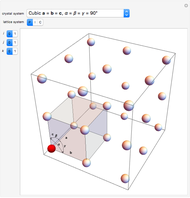
Snapshot 1: dissociation of 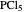
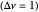 : As required by the Le Chatelier principle, the increase in pressure produces a decrease in the total moles inside the reactor for this specific reaction. In this case, this tendency occurs by shifting toward the side of reactants.
: As required by the Le Chatelier principle, the increase in pressure produces a decrease in the total moles inside the reactor for this specific reaction. In this case, this tendency occurs by shifting toward the side of reactants.
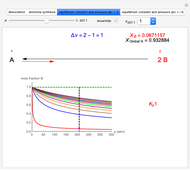
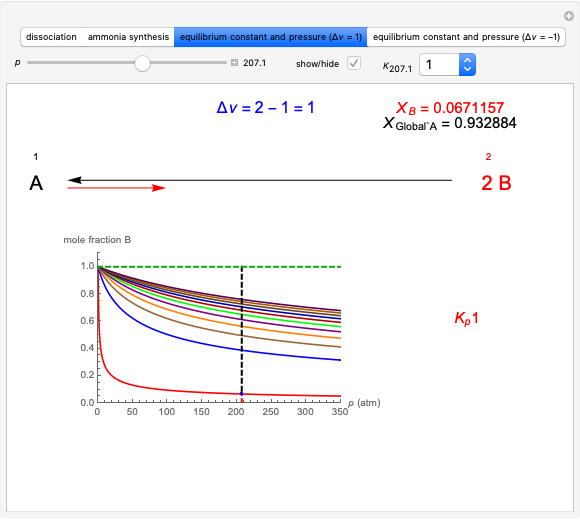
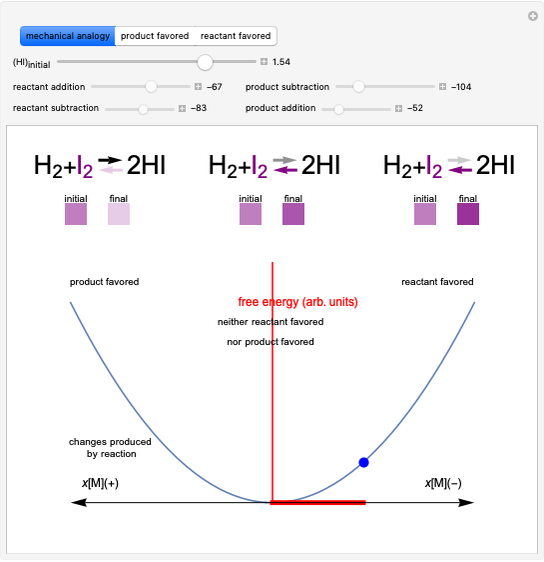
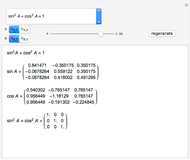
Snapshot 2: generic dissociation reaction 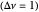 : Shifting toward the side of reactants is also influenced by the value of
: Shifting toward the side of reactants is also influenced by the value of  ; the increase in
; the increase in 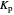 and pressure act in the opposite way. With the shift of the equilibrium to the left, the mole fraction of
and pressure act in the opposite way. With the shift of the equilibrium to the left, the mole fraction of 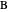
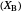 decreases.
decreases.
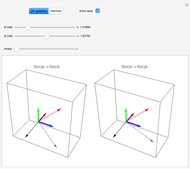
Snapshot 3: generic dimerization reaction 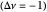 : Both the value of
: Both the value of 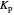 and the pressure shift the chemical equilibrium toward the side of products; in this way the molar fraction of
and the pressure shift the chemical equilibrium toward the side of products; in this way the molar fraction of 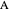
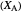 grows.
grows.
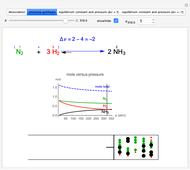
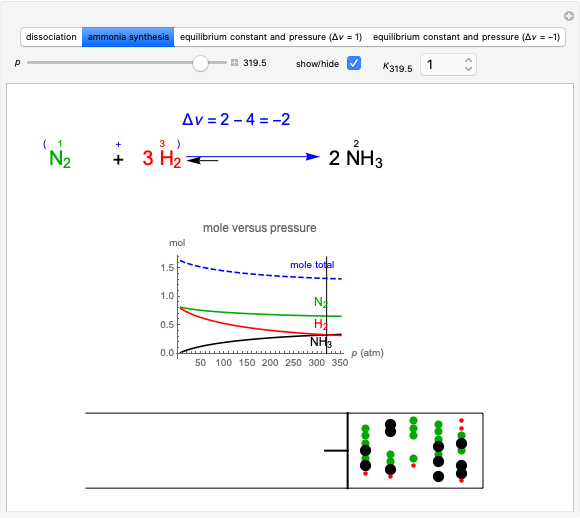
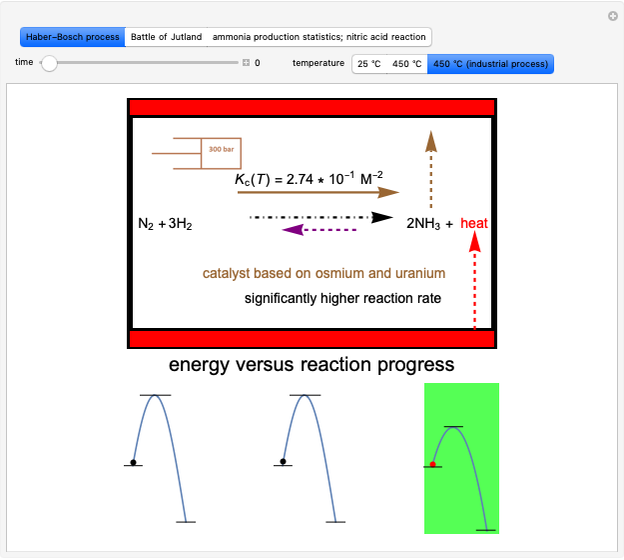
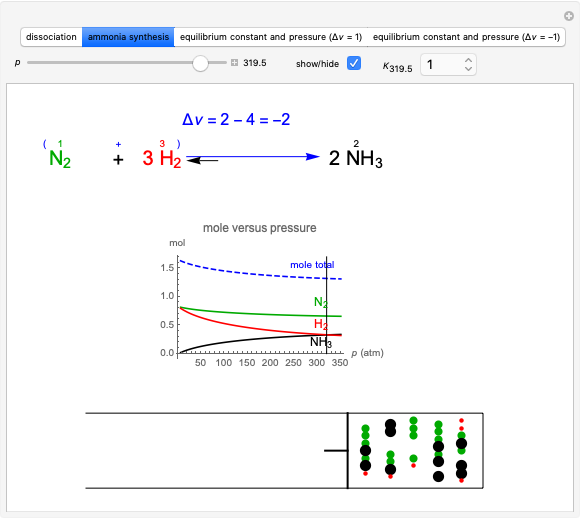
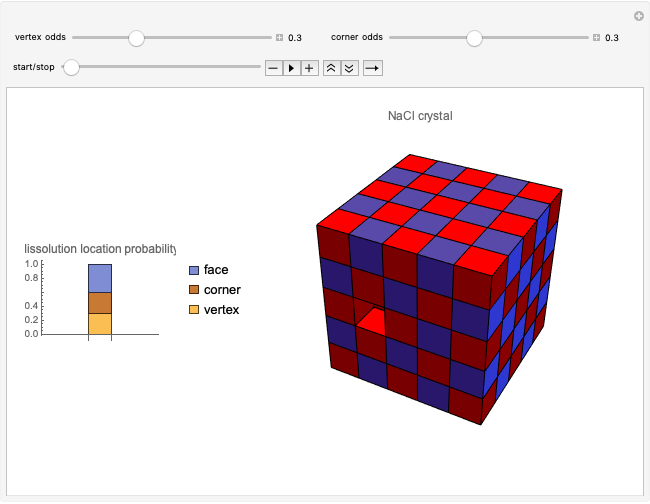
Snapshot 4: synthesis of ammonia in the Haber–Bosch process 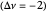 : As required by the Le Chatelier principle, the increase in pressure produces a decrease in the total moles inside the reactor. The trend can be easily visualized by representing the different molecules as indistinguishable particles and is achieved by shifting the reaction toward the side of products.
: As required by the Le Chatelier principle, the increase in pressure produces a decrease in the total moles inside the reactor. The trend can be easily visualized by representing the different molecules as indistinguishable particles and is achieved by shifting the reaction toward the side of products.
References
[1] P. M. Lausarot and G. A. Vaglio, Stechiometria per la Chimica generale, Padova, Italy: Piccin Nuova Libraria S.p.A., 2005.
[2] La sintesi dell'ammoniaca-Online Scuola Zanichelli. (Sep 2, 2020) online.scuola.zanichelli.it/scopriamolachimica-files/Schede/Zanichelli_Bagatti_Scopriamo _Cap09_S_Ammoniaca.pdf.
[3] P. Atkins and J. de Paula, Atkins' Physical Chemistry, 8th ed., New York: Oxford University Press, 2006.
Permanent Citation