Cyclic Compound Formation via Diels-Alder Type Reactions

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
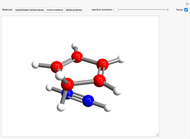
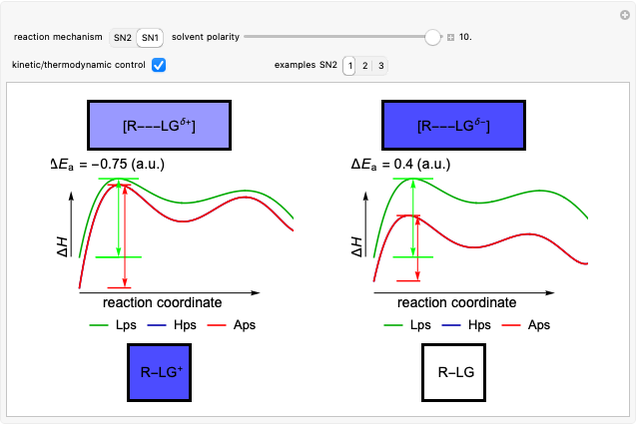
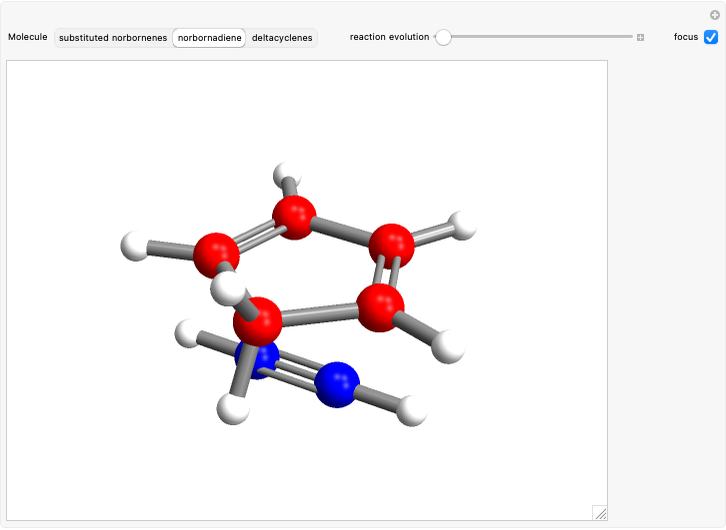
This Demonstration considers the Diels–Alder reaction [4+2] leading to the formation of bicyclic compounds [1].
[more]
Contributed by: D. Meliga, V. Giambrone, L. Lavagnino and S. Z. Lavagnino (October 12)
With additional contributions by: F. Calcagno (ITIS A. Artom, Asti)
Open content licensed under CC BY-NC-SA
Details
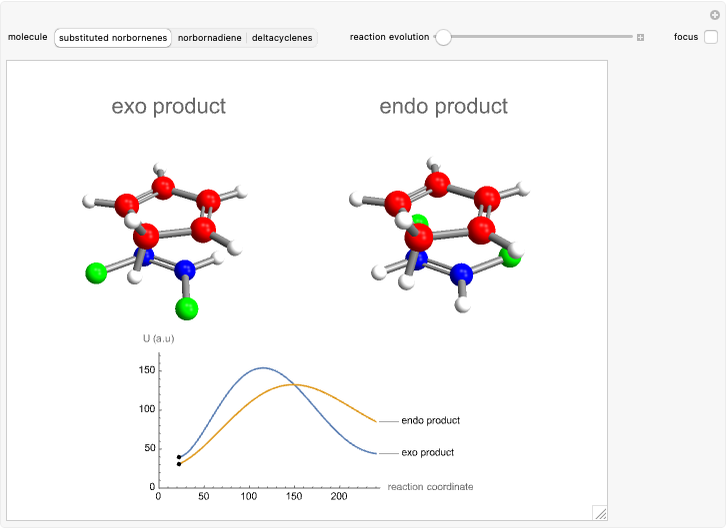
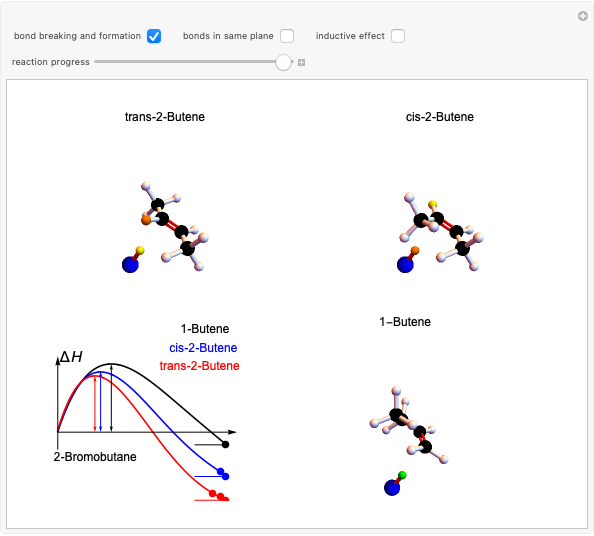
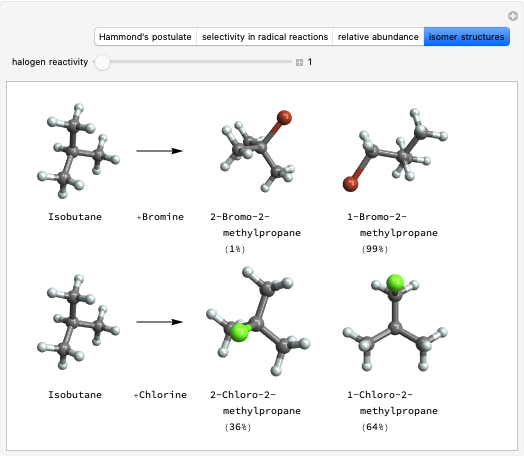
Snapshot 1: formation of the two isomers of the substituted norbornene; note that intramolecular interactions favor the endo isomer by lowering the activation energy
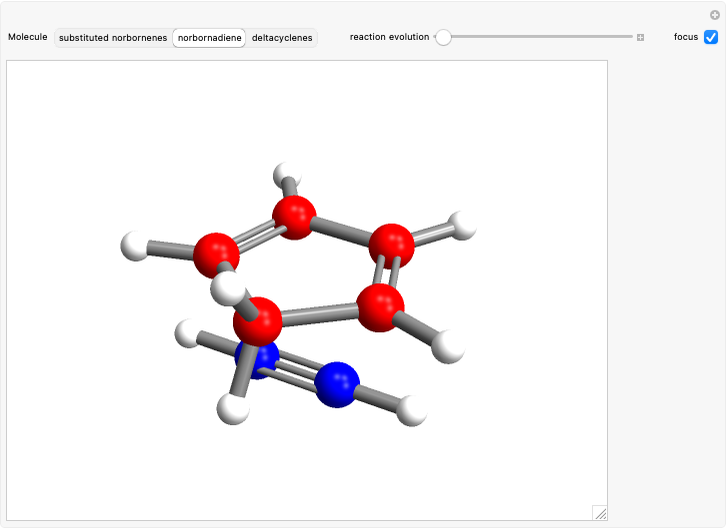
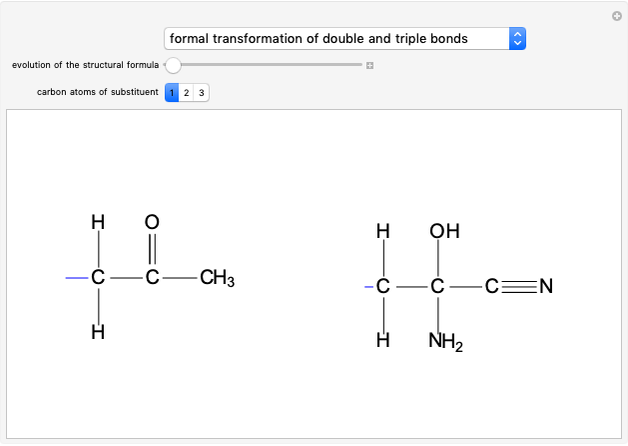
Snapshot 2: formation of the norbornadiene, in which the initial triple bond transforms into a double bond
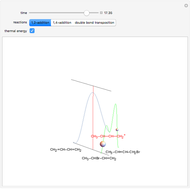
Snapshot 3: formation of deltacyclenes is not classified as a Diels–Alder reaction, but analogies exist since a 6-carbon ring is also formed
References
[1] S. Z. Lavagnino, Diels Alder Endo Exo [Video]. (Aug 14, 2023) www.youtube.com/watch?v=N_-UdOBfWf0&list=PLswwssc6Q2yYoP_INHmbmouyxW8oP _Gib&index=60.
[2] Exo vs Endo Products In The Diels Alder: How To Tell Them Apart. https://www.masterorganicchemistry.com/2018/02/09/endo-exo-diels-alder-telling-them-apart.
[3] Norbornadiene. https://en.wikipedia.org/wiki/Norbornadiene.
[4] A. Pounder, L. D. Chen and W. Tam, "Ruthenium-Catalyzed [2 + 2] versus Homo Diels−Alder [2 + 2 + 2] Cycloadditions of Norbornadiene and Disubstituted Alkynes: A DFT Study," ACS Omega, 6(1), 2021 pp. 900−911. doi:10.1021%2Facsomega.0c05499.
Snapshots
Permanent Citation