Hammond's Postulate and Selectivity in Free Radical Halogenation

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
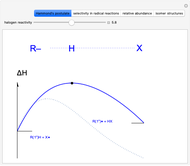
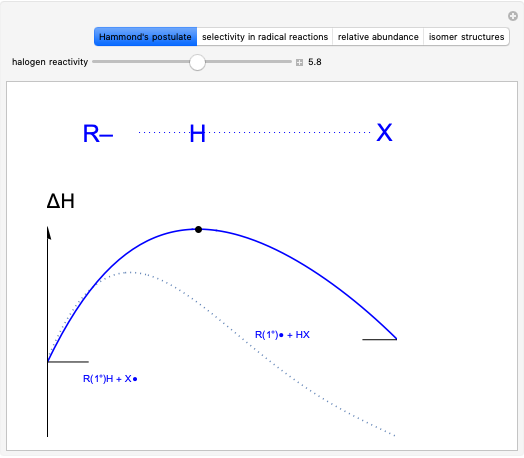
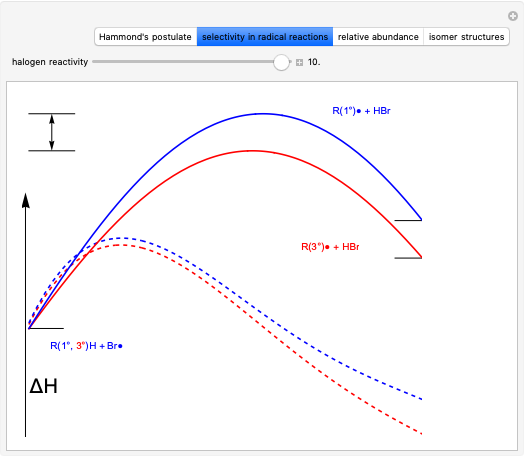
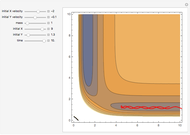
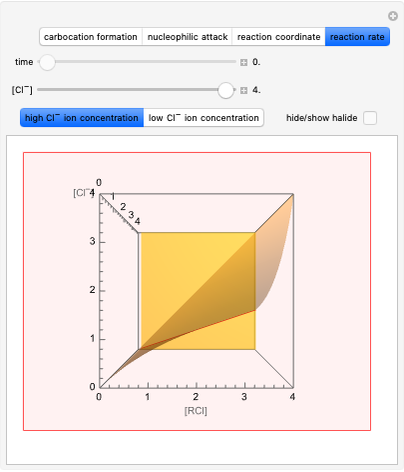
This Demonstration explores why bromination is more selective than chlorination, as a consequence of Hammond's postulate. We introduce a generic halogen X varied between chlorine and bromine. Select "Hammond's postulate" to see the reaction coordinate for the chlorination on primary carbon R(1°)H; in this case, the transition state resembles the reactant more closely and the reaction is exothermic. By contrast, the transition state in bromination is closer to the product, so the reaction is endothermic.
[more]
Contributed by: D. Meliga, A. Ratti, L. Lavagnino and S.Z. Lavagnino (August 2022)
Open content licensed under CC BY-NC-SA
Snapshots
Details
Snapshot 1: by Hammond's postulate, a reaction with a transition state closer to the reactant is exothermic (e.g. reaction with chlorine) while a transition state closer to the products leads to an endothermic reaction (e.g. reaction with bromine).
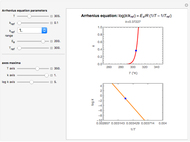
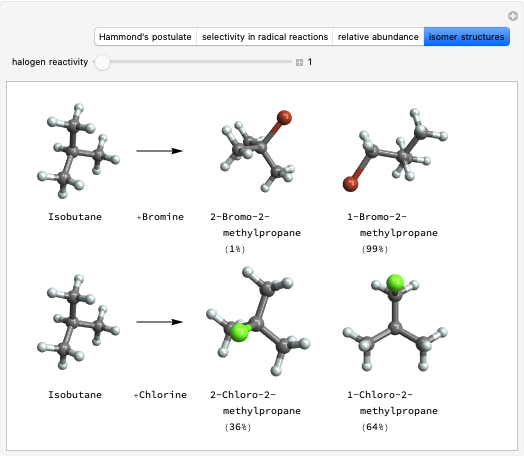
Snapshot 2: Hammond's postulate implies greater selectivity in bromination compared to chlorination; this happens because the activaction energy gap between primary and tertiary is wider in bromination than in chlorination.
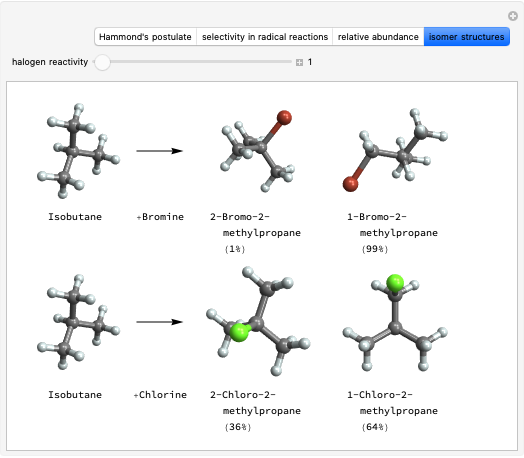
Snapshot 3: comparing the statistical prediction for bromination with the experimental results confirms Hammond's postulate.
References
[1] W. H. Brown, B. L. Iverson, E. V. Anslyn and C. S. Foote, Organic Chemistry, 7th ed., Belmont, CA: Wadsworth Cengage Learning, 2014.
[2] J. Ashenhurst. "Selectivity in Free Radical Reactions: Bromination vs. Chlorination." (Apr 13, 2022) www.masterorganicchemistry.com/2013/10/31/selectivity-in-free-radical-reactions-bromine-vs-chlorine.
Permanent Citation