Symmetry Planes and Stereoisomers

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
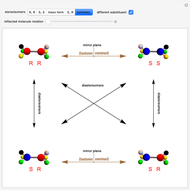
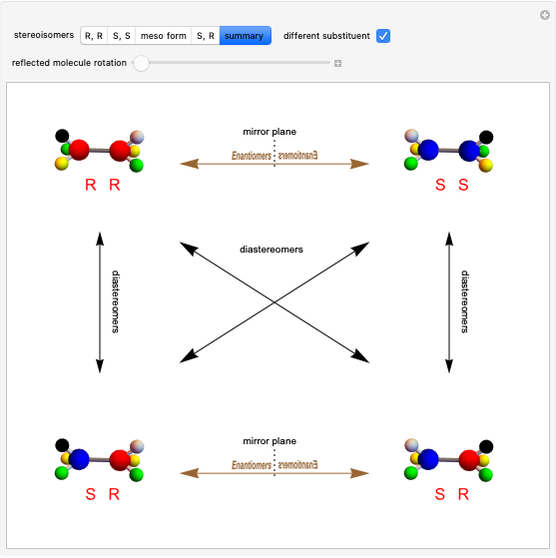
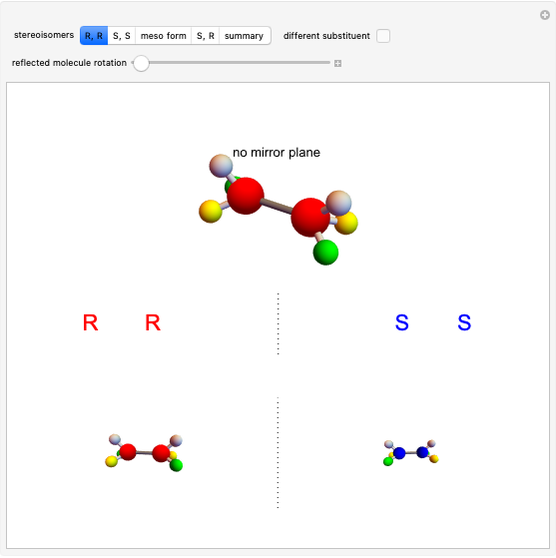
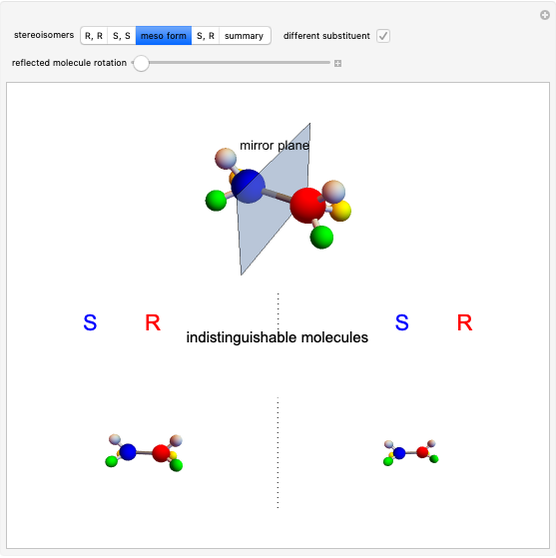
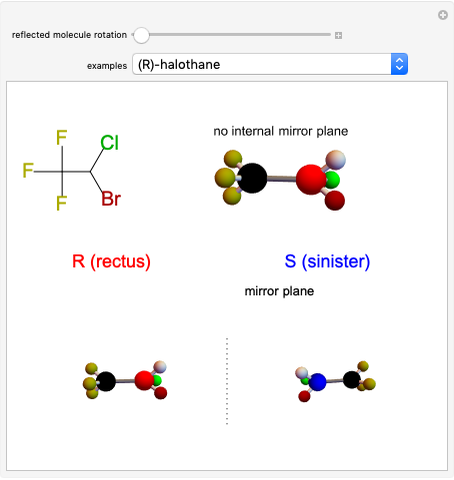
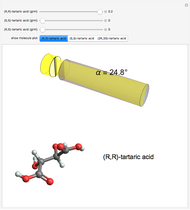
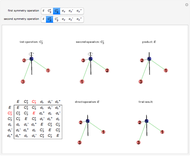
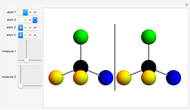
Molecules with the same molecular formula but a different spatial arrangement are classified as stereoisomers. These can be either enantiomers or diastereomers. Two molecules are called enantiomers if they are mirror images. They are diastereomers if no plane of reflection exists. Diastereomers can have different physical properties and reactivities. In this Demonstration, the molecules considered have two chiral centers ( ), thus the maximum number of stereoisomers is equal to 4 (
), thus the maximum number of stereoisomers is equal to 4 ( ), or 3 if the two chiral centers are identical. A meso form has two chiral centers but is superposable with its mirror image and thus optically inactive.
), or 3 if the two chiral centers are identical. A meso form has two chiral centers but is superposable with its mirror image and thus optically inactive.
Contributed by: D. Meliga, A. Ratti, L. Lavagnino and S. Z. Lavagnino (August 2022)
Open content licensed under CC BY-NC-SA
Snapshots
Details
Permanent Citation