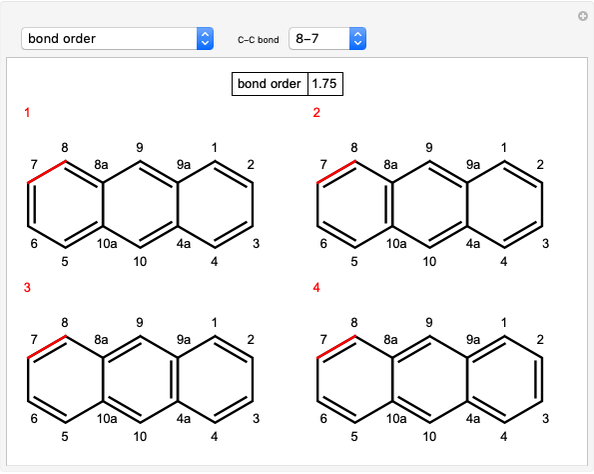
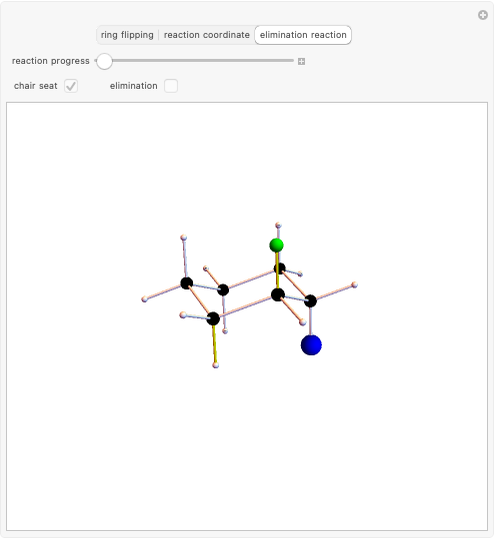
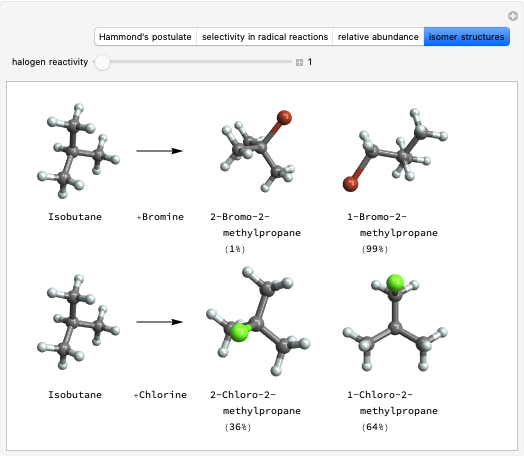
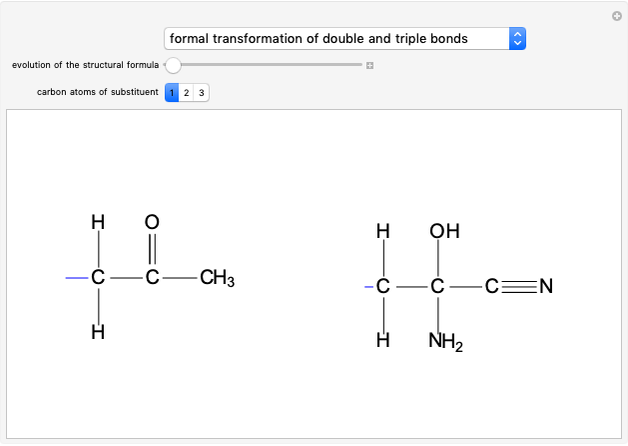
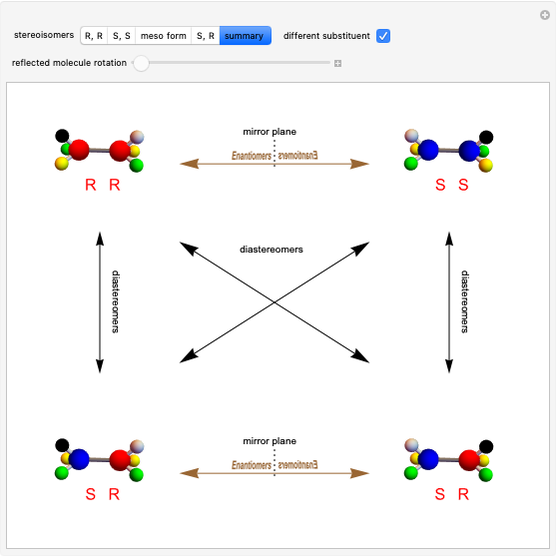
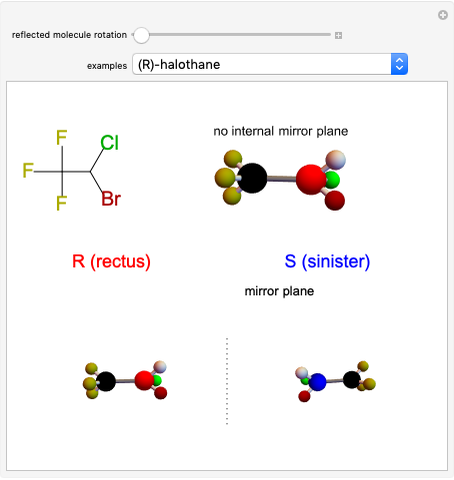
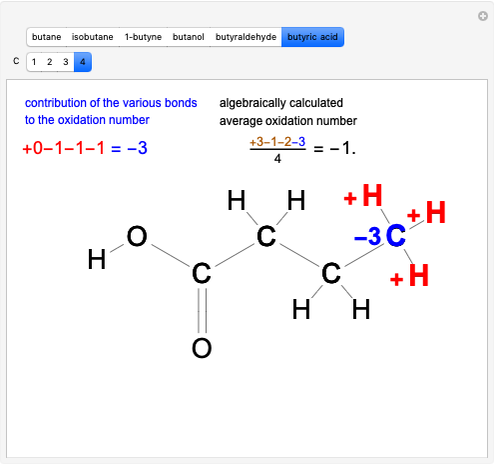
Stereochemistry of E2 Elimination Reactions

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
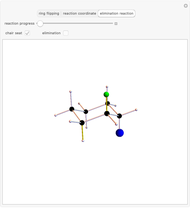
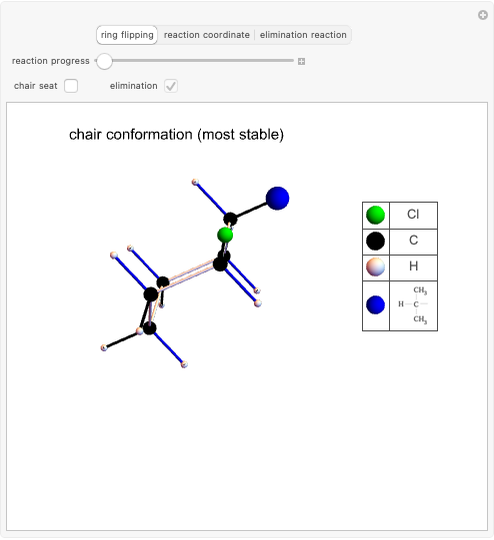
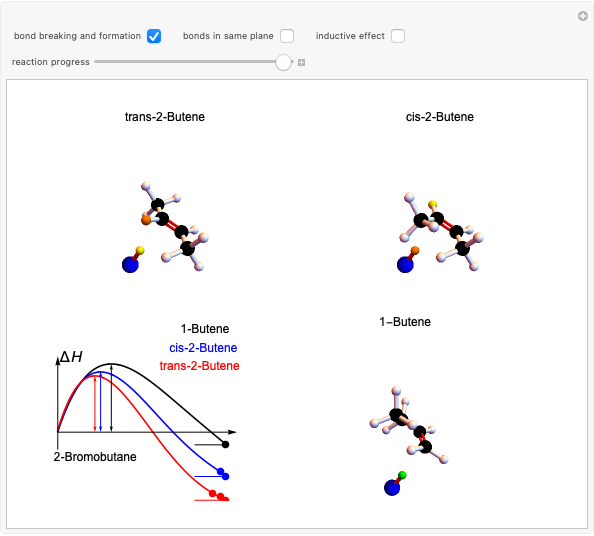
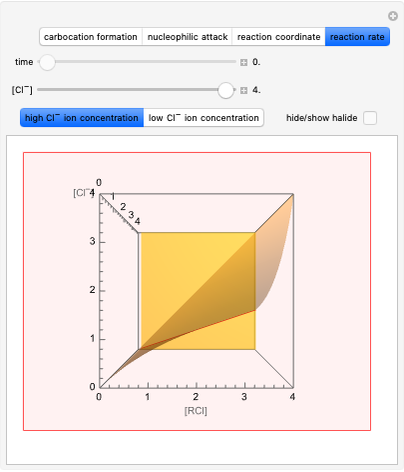
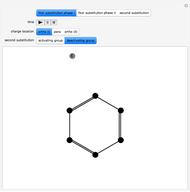
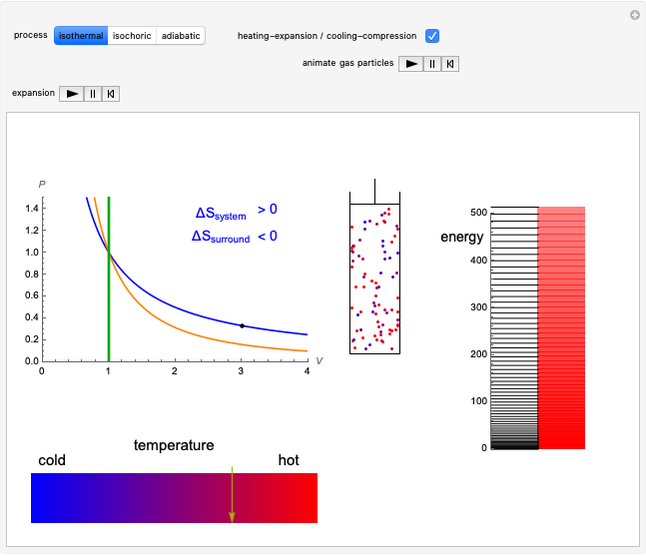
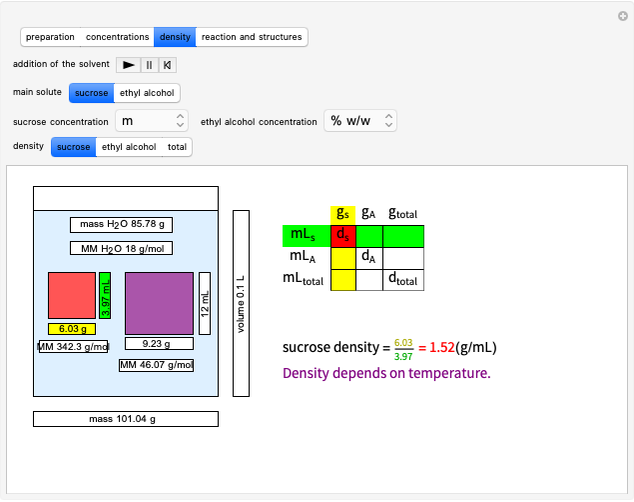
This Demonstration considers the different conformations of a substituted cyclohexane, trans-1-chloro-2-isopropyl-cyclohexane, in the course of ring flipping and elimination reactions. Two different chair conformations are stable, the more stable being the one with the lower steric bulk. Select "ring flipping" and the "reaction progress" slider to observe the transition between the two chair conformations [1]. In this transition the equatorial and axial ligands interchange. Select "chair seat" to see the common seat in the two chair conformations.
[more]
Contributed by: D. Meliga, V. Giambrone, L. Lavagnino and S. Z. Lavagnino (January 2023)
Open content licensed under CC BY-NC-SA
Snapshots
Details
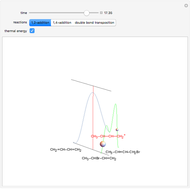
Snapshot 1: substituted cyclohexane change from a more stable configuration to a less stable one as a consequence of steric effects
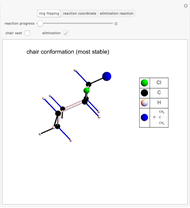
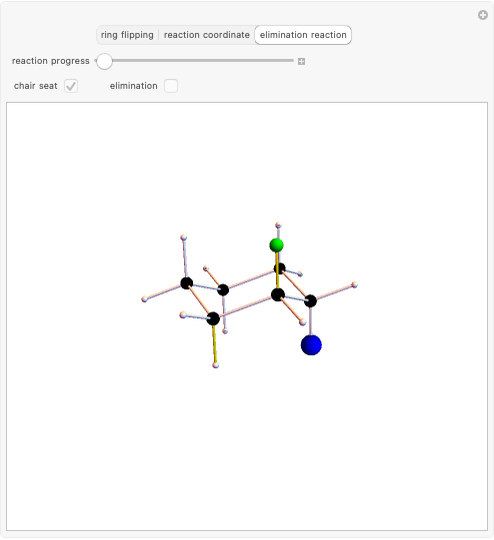
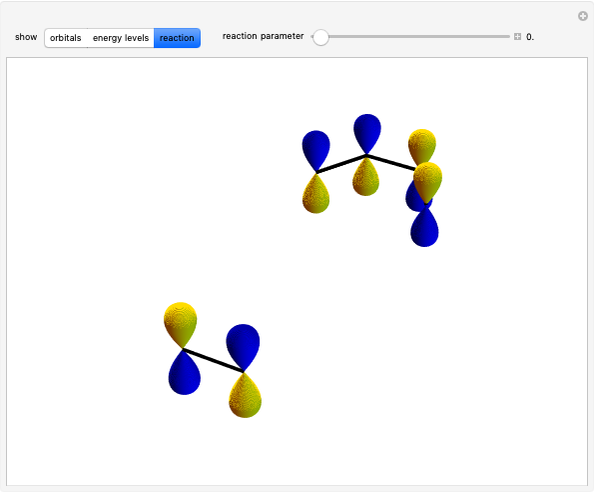
Snapshot 2: the leaving group (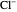 ) is in anti-periplanar position to the hydrogen and after elimination a double bond is formed
) is in anti-periplanar position to the hydrogen and after elimination a double bond is formed
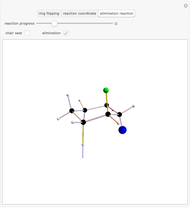
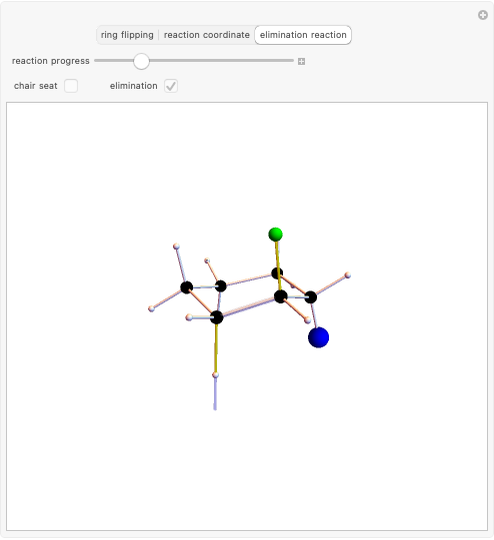
Snapshot 3: Elimination reaction further along
References
[1] J. Ashenhurst. "The Cyclohexane Chair Flip–Energy Diagram." (Nov 18, 2022) www.masterorganicchemistry.com/2014/06/06/the-cyclohexane-chair-flip-energy-diagram.
[2] X. Liu, "4.3 Conformation Analysis of Cyclohexane," Organic Chemistry I, (Nov 18, 2022) kpu.pressbooks.pub/organicchemistry/chapter/4-3-conformation-analysis-of-cyclohexane-and-substituted-cyclohexanes.
[3] S. V. Lavagnino. Eliminazione secondo Hofmann [Video]. (Nov 18, 2022) www.youtube.com/watch?v=nZDIQsNCpjM&list=PLswwssc6Q2yYoP_INHmbmouyxW8oP_Gib&index=46.
[4] J. Ashenhurst. "Antiperiplanar Relationships: The E2 Reaction and Cyclohexane Rings." (Nov 18, 2022) www.masterorganicchemistry.com/2012/10/18/the-e2-reaction-and-cyclohexane-rings.
Permanent Citation