Nucleophilic Substitution (SN2) Reactions

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
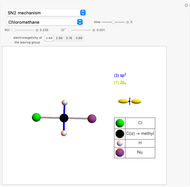
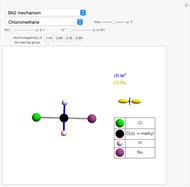
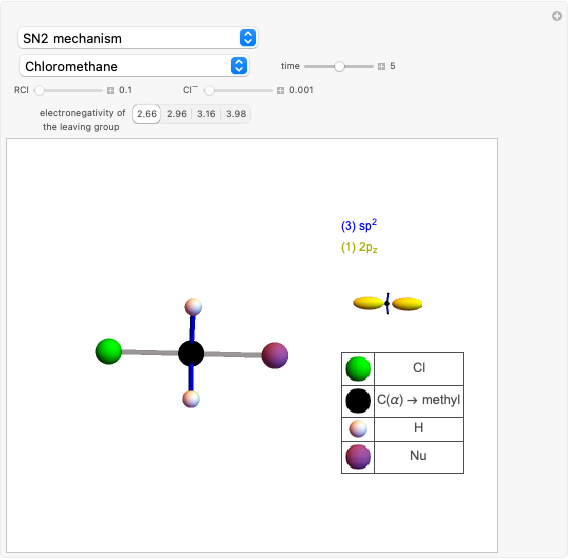
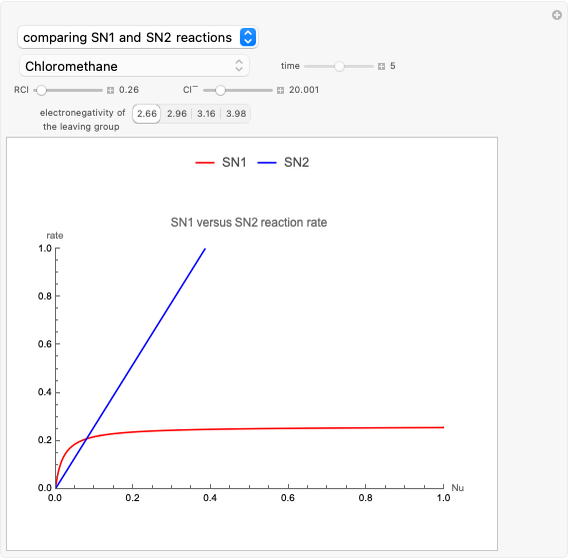
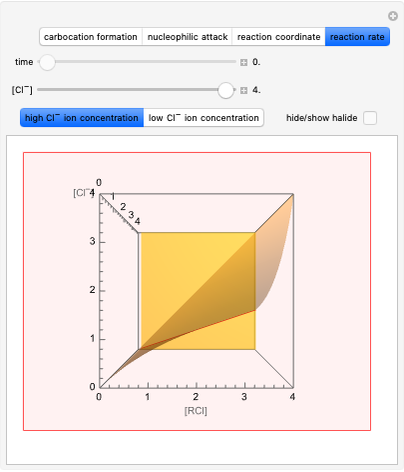
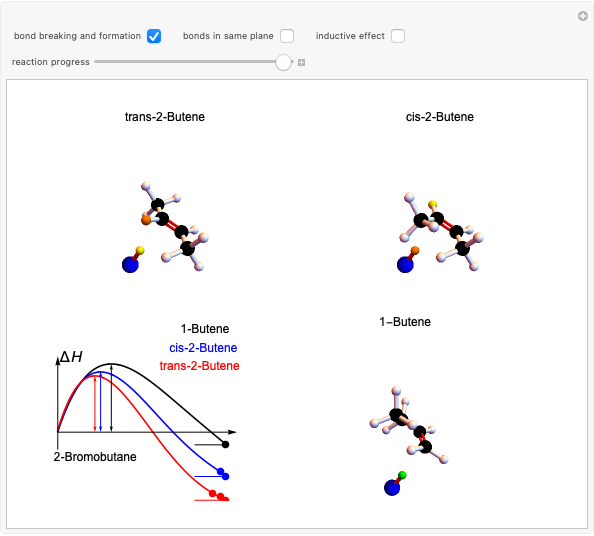
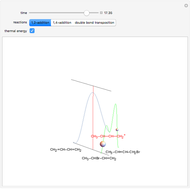
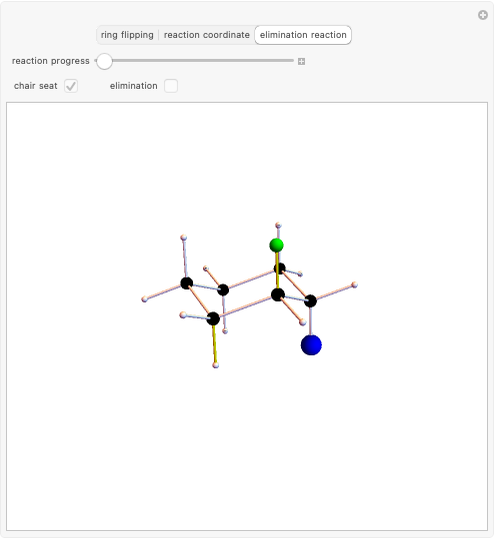
This Demonstration discusses the main features of nucleophilic substitution reactions of alkyl halides, which take place in a single concerted stage. These are known as SN2 reactions.
[more]
Contributed by: D. Meliga, V. Giambrone, L. Lavagnino and S. Z. Lavagnino (January 2023)
Open content licensed under CC BY-NC-SA
Snapshots
Details
Snapshot 1: C-Cl bond is about to break, with early formation of the C-Nu bond
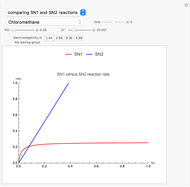
Snapshot 2: slopes of the reaction rates are different for the two types of reactions
Snapshot 3: increasing electronegativity increases the activation energy of the reaction
References
[1] H. Hart, L. E. Craine and D. J. Hart, Organic Chemistry: A Short Course, 10th ed., Boston: Houghton Mifflin, Co., 1999.
[2] S. V. Lavagnino. SN1 e SN2 [Video]. (Aug 23, 2022) www.youtube.com/watch?v=jZPElbt_-9I.
Permanent Citation