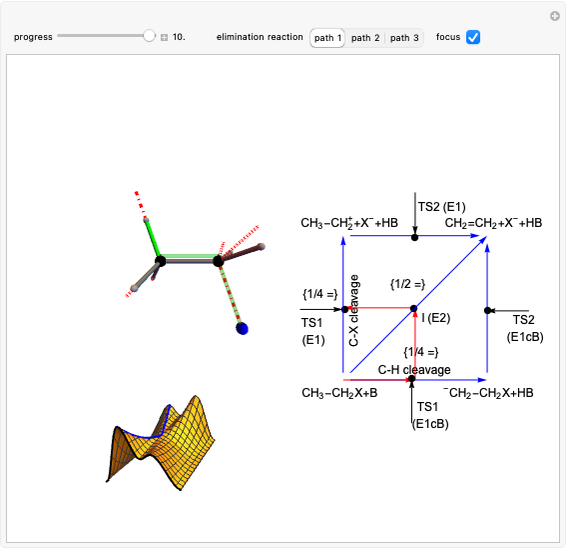
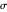This Demonstration considers various aspects of the Diels–Alder (DA) reaction.
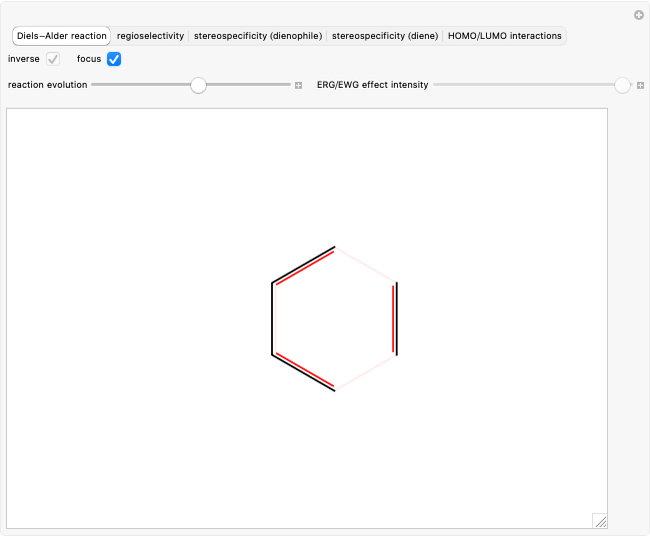
Select "Diels–Alder reaction" to observe how a diene (1,3-butadiene) can react with a dienophile (ethylene) to form a six-carbon ring with a double bond: first the diene assumes the s-cis configuration (thermodynamically unstable compared to the s-trans one), and then three bonds  are replaced with three new bonds: two simple bonds
are replaced with three new bonds: two simple bonds  and a new double bond, all in a single stage.
and a new double bond, all in a single stage.
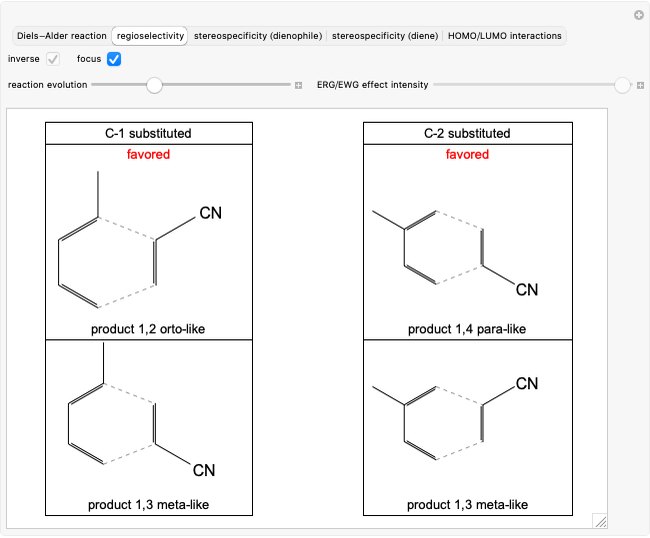
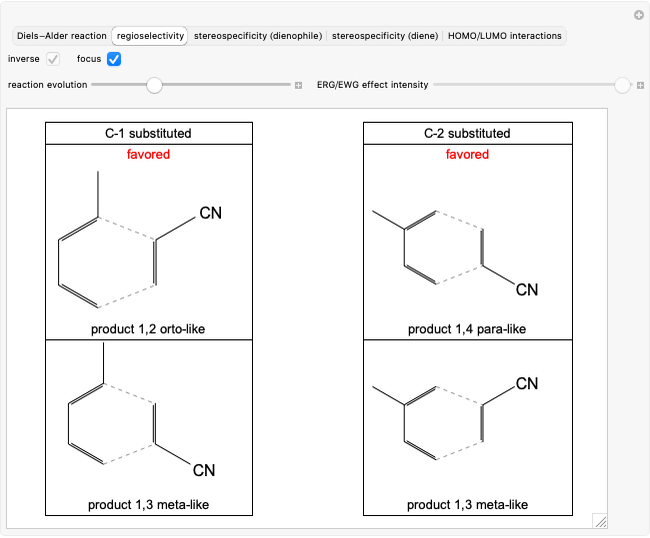
Select "regioselectivity" for the mono-substituted dienes and dienophiles case and obtain two products that are structural isomers between them. Since the reaction takes place under kinetic control [1], the reaction can be considered regiospecific and two isomers are preferentially formed.
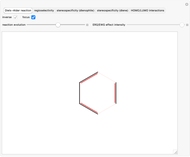
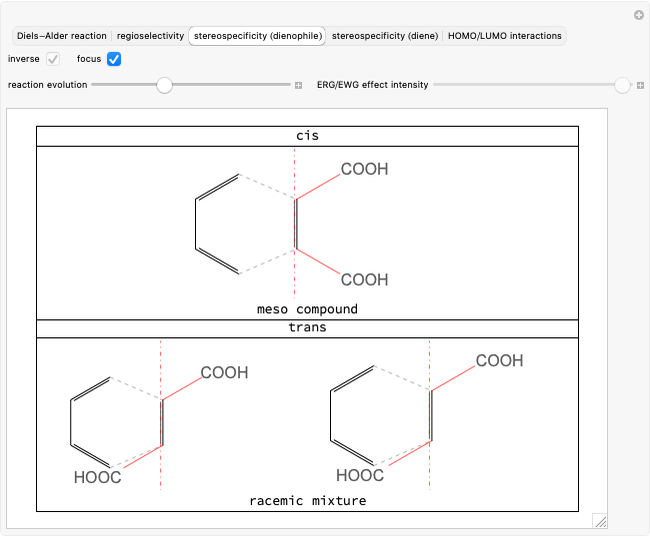
The DA reaction is a syn addition [1] whereby the stereochemistry of both the dienophile and the diene is preserved in the products. The cis or trans conformation of the dienophile, associated with the double bond, is maintained in the substituted cyclohexene but this time it is referred to as the molecular plane. Select "stereospecificity (dienophile)" to highlight the fact that starting from cis dienophiles, meso products are formed, while with trans dienophiles, a racemic mixture is obtained.
Select "stereospecificity (diene)" to see the same preservation of isomerism applied to dienes, but the meso compound is obtained from a dienophile of the trans, trans-type and the racemic mixture with the trans dienophile, cis-.
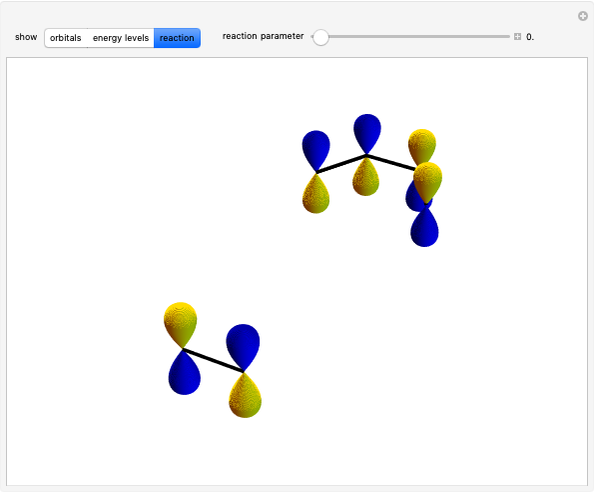
Select "HOMO/LUMO interactions" to show the frontier molecular orbitals (FMO); without any substituents, two possible interactions (double-headed arrows help visualization) can happen. By changing substituents (EWG, ERG) with "ERG/EWG effect intensity," one interaction becomes dominant (shorter arrow), and with "inverse" it becomes possible to swap effects between the diene and the dienophile.
Select "focus" to highlight the bonds that are most involved in the reaction.
[less]