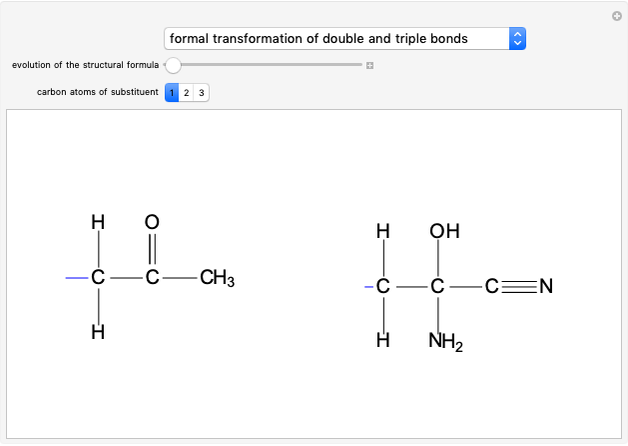
Symmetry Planes and Optical Isomerism

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
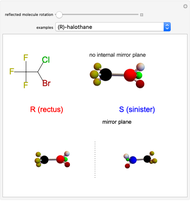
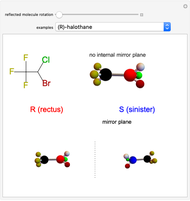
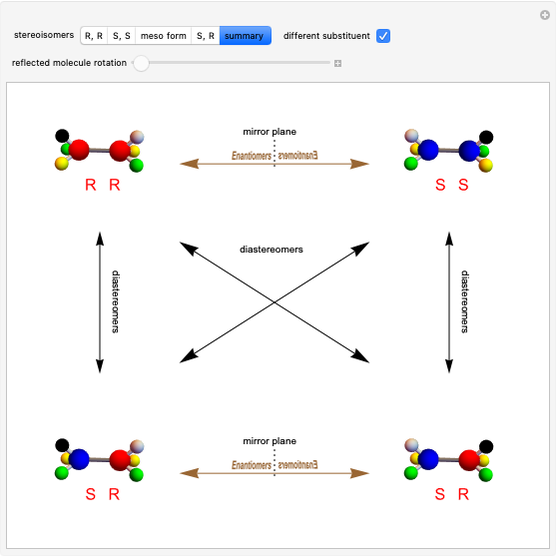
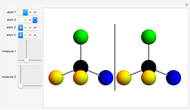
This Demonstration shows that if a molecule possesses a symmetry plane, the molecule cannot be chiral and its mirror image is indistinguishable. [1]
[more]
Contributed by: D. Meliga, A. Ratti, L. Lavagnino and S. Z. Lavagnino (August 2022)
Open content licensed under CC BY-NC-SA
Snapshots
Details
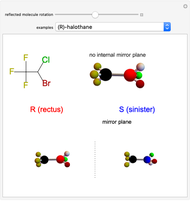
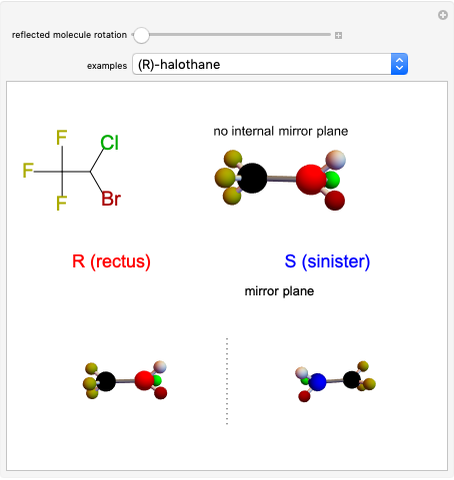
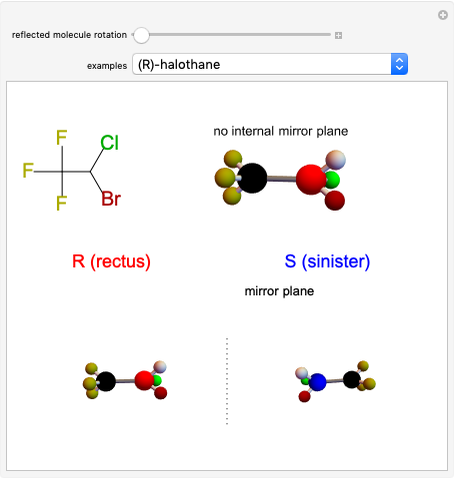
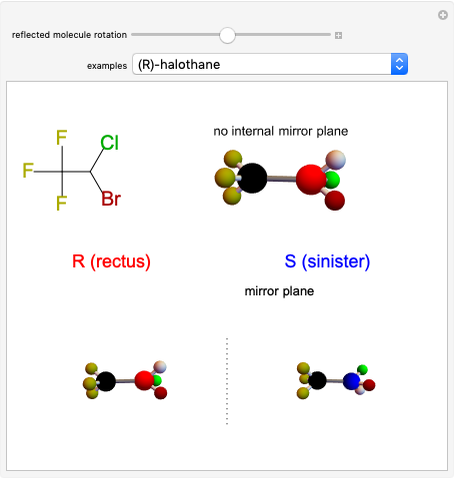
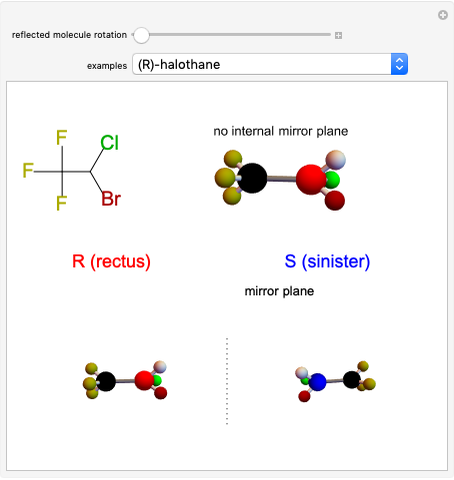
Snapshot 1: halothane is a chiral molecule; its mirror image is not superposable
Snapshot 2: no rotation can accomplish superposition of the two enantiomers, which are distinct molecules
Snapshot 3: 1,1-dichloroethane is not a chiral molecule, since two substituents are identical. Reflection produces the identical molecule, as does 180-degree rotation of the mirror image.
Snapshot 4: 1-chloro-2-fluoro-1-iodopropadiene is a chiral molecule without chiral centers
References
[1] "4.1 Chirality," Organic Chemistry 1: An Open Textbook. (Jan 10, 2022) courses.lumenlearning.com/suny-potsdam-organicchemistry/chapter/4-1-chirality.
[2] H. Hart, L. E. Craine and D. J. Hart, Organic Chemistry: A Short Course, 10th ed., Boston: Houghton Mifflin, Co., 1999.
Permanent Citation