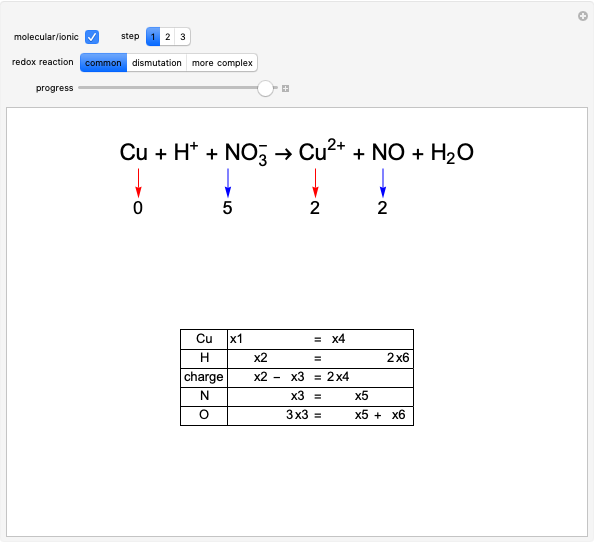
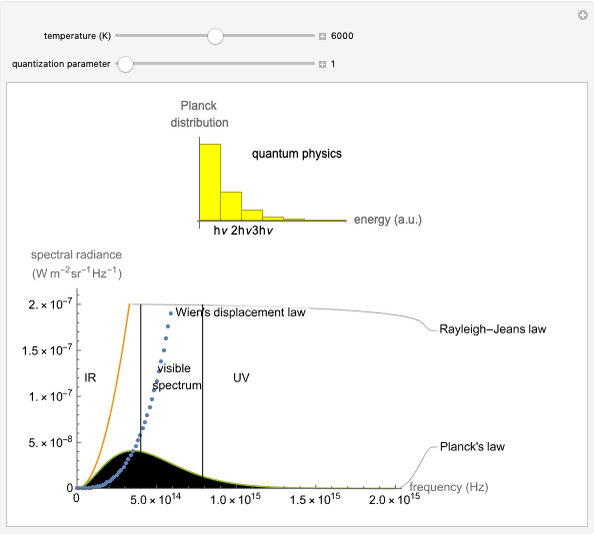
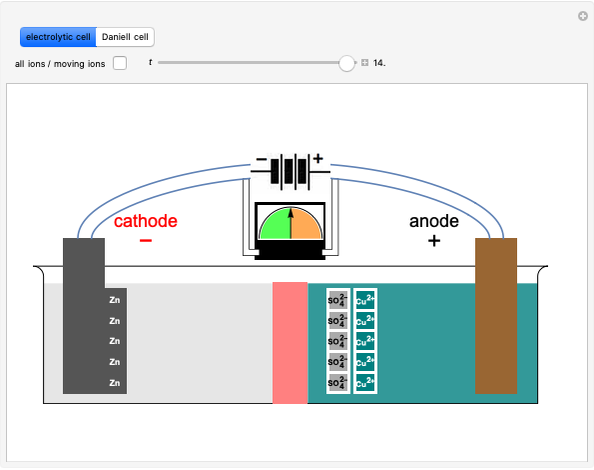
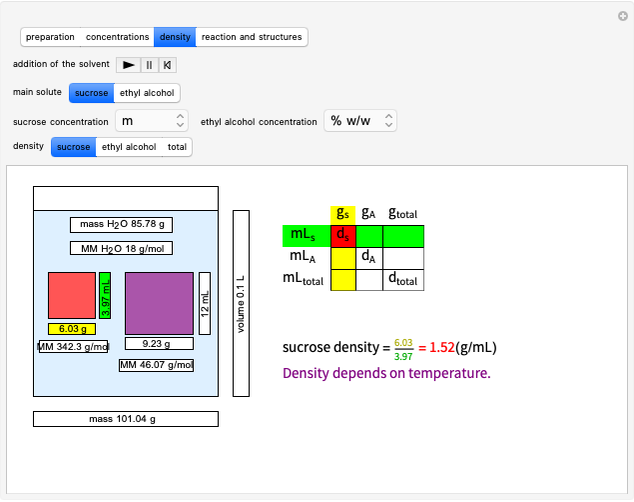
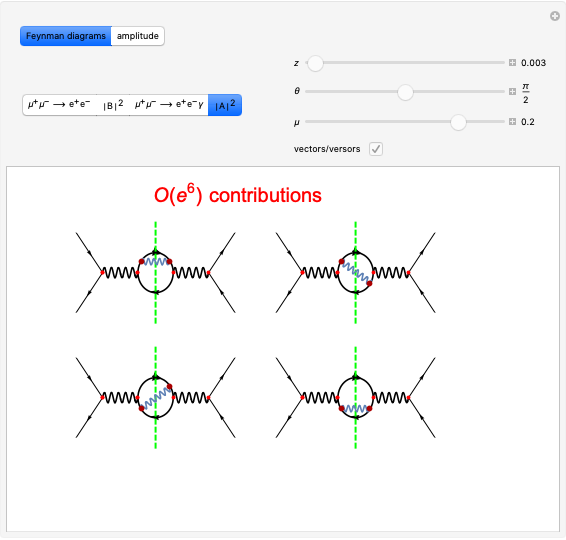
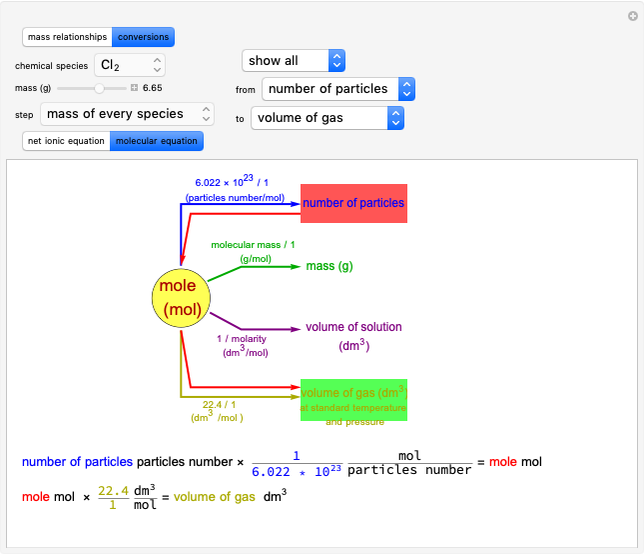
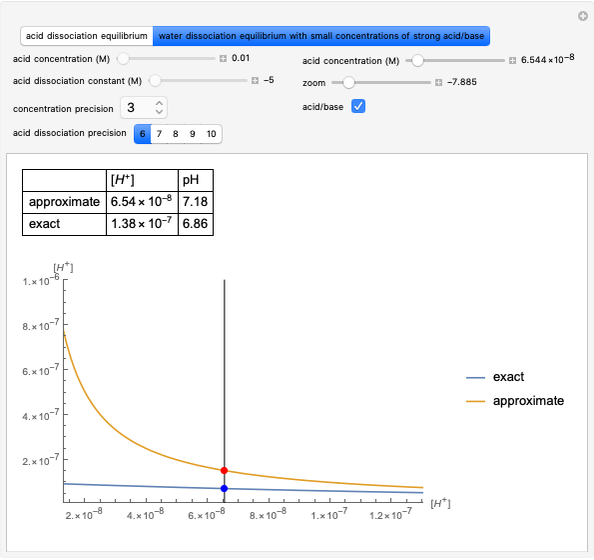
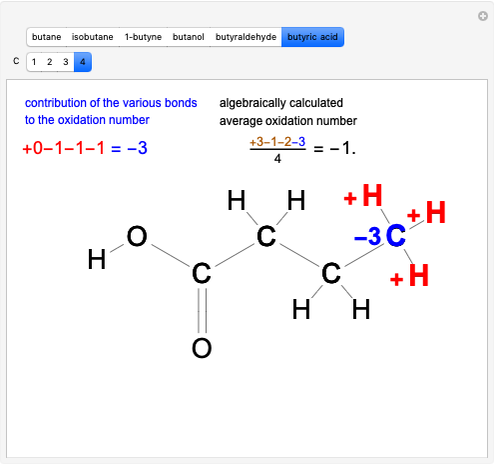
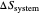Boltzmann's Analysis of Macroscopic and Microscopic Aspects of Reversible Thermodynamic Processes

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
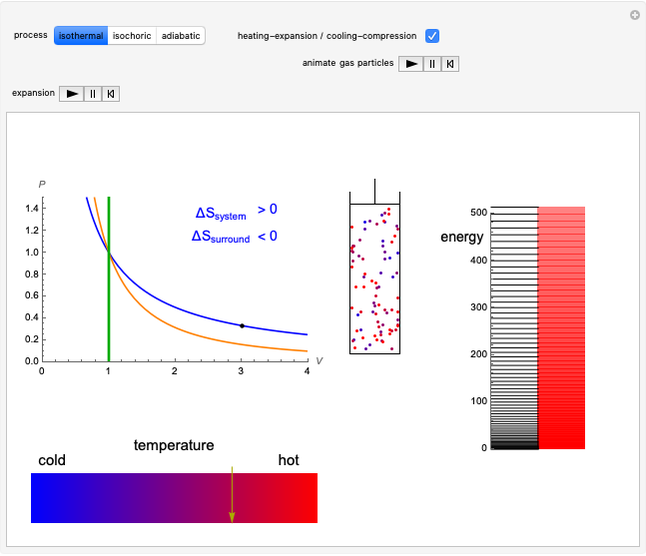
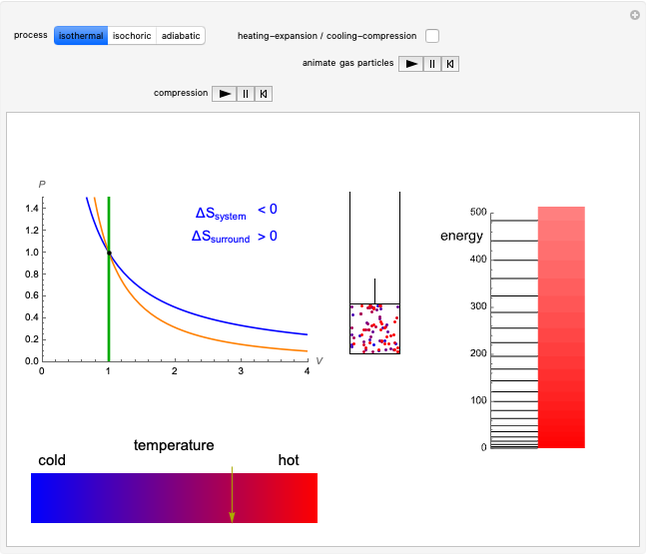
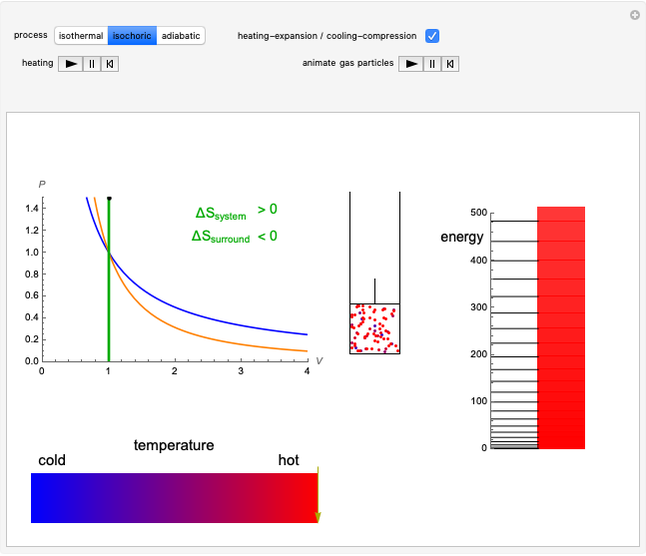
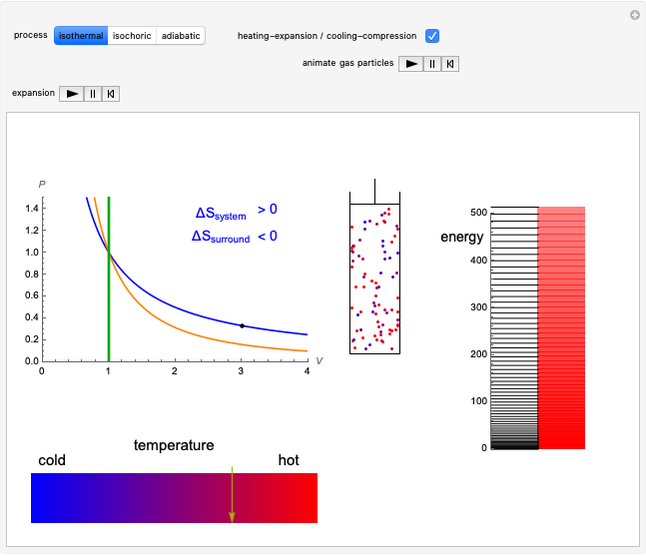
This Demonstration considers three different reversible processes from both a macroscopic and a microscopic point of view: isothermal expansion and compression, isochoric heating and cooling, and adiabatic expansion and compression [1]. Select the process, then use the checkbox to choose between heating–expansion or cooling–compression.
[more]
Contributed by: D. Meliga, A. Ratti, L. Lavagnino and S. Z. Lavagnino (August 2022)
Open content licensed under CC BY-NC-SA
Snapshots
Details
Snapshot 1: Isothermal expansion. At constant temperature, expanding the volume causes a compression in the energy-level spacing. As a consequence, more levels are occupied and the system entropy increases  ).
).
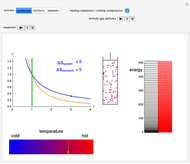
Snapshot 2: Isochoric heating. As the energy levels remain unchanged, rising temperature causes increased occupation of higher energy levels. The system entropy thus increases  ).
).
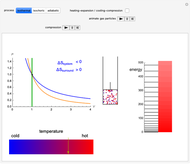
Snapshot 3: Adiabatic expansion. Expansion in volume causes a decreased spacing of the levels. A decrease in temperature leads to decreased occupancy of higher levels. The two effects compensate and the entropy remains constant 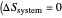 ,
,  ).
).
Reference
[1] P. Atkins and L. Jones, Chemical Principles: The Quest for Insight, New York: W. H. Freeman, 1999.
Permanent Citation