Williams, Landel, and Ferry Equation Compared with Actual and "Universal" Constants

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
Contributed by: Mark D. Normand and Micha Peleg (February 2013)
Open content licensed under CC BY-NC-SA
Snapshots
Details
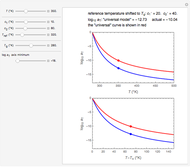
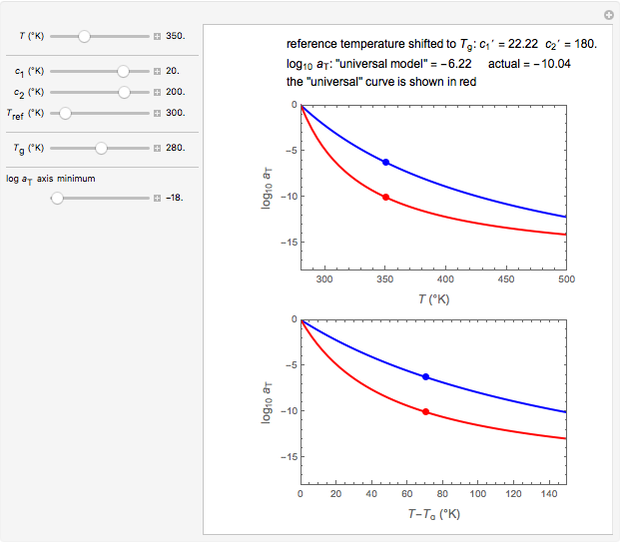
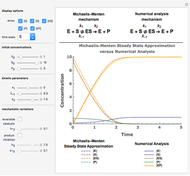
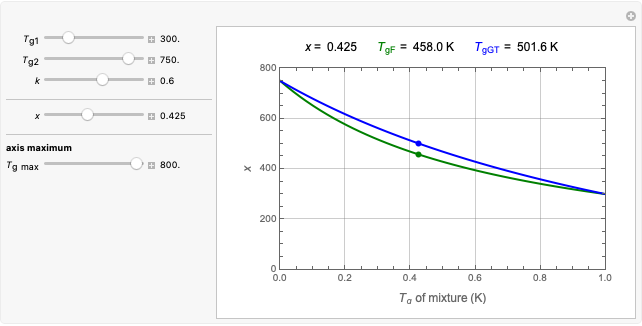
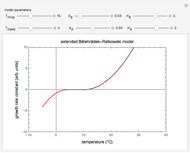
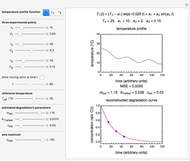
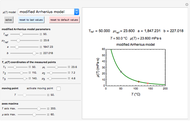
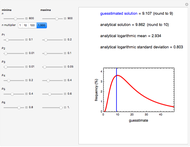
Snapshot 1:  versus
versus  where
where  and
and 
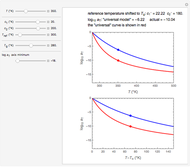
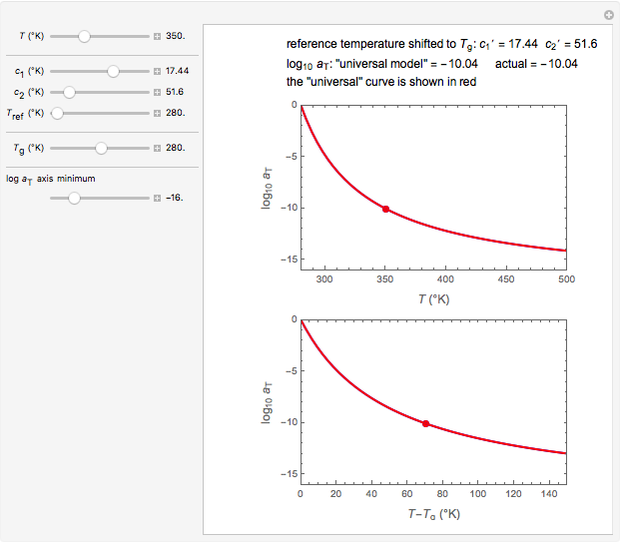
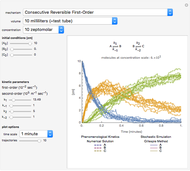
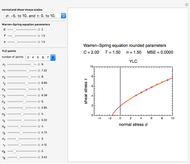
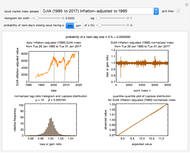
Snapshot 2:  versus
versus  where
where  and
and 
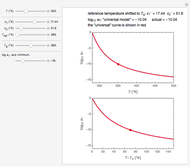
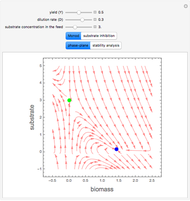
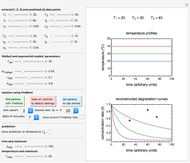
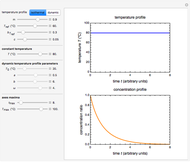
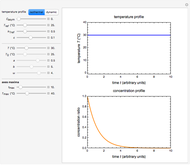
Snapshot 3:  versus
versus  where
where  and
and  and
and 
The original WLF model can be written as  , where
, where  is the shift factor,
is the shift factor,  is a reference temperature in °K, and
is a reference temperature in °K, and  and
and  are experimentally determined constants (
are experimentally determined constants ( is dimensionless and
is dimensionless and  is in °K). When the arbitrary reference temperature is shifted to the glass transition temperature
is in °K). When the arbitrary reference temperature is shifted to the glass transition temperature  , the equation becomes
, the equation becomes  , where
, where  and
and  , with
, with  [1].
[1].
The WLF model's authors suggested that in the absence of experimental data one could use  and
and  as first approximations. These values were derived by averaging the experimental values of several rubbery polymers and are widely known as the WLF model's universal constants.
as first approximations. These values were derived by averaging the experimental values of several rubbery polymers and are widely known as the WLF model's universal constants.
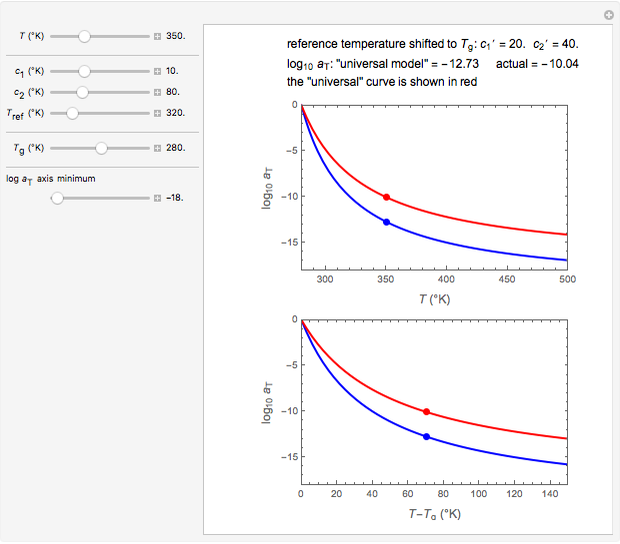
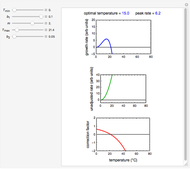
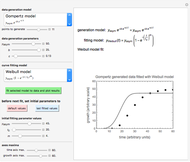
In this Demonstration, experimentally determined constants  and
and  , the reference temperature
, the reference temperature  , and the polymers or food's glass transition temperature
, and the polymers or food's glass transition temperature  are entered with sliders. The program then calculates the corresponding
are entered with sliders. The program then calculates the corresponding  and
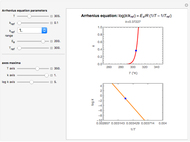
and 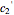 and displays, on the same graph, the resulting
and displays, on the same graph, the resulting  versus
versus  relationship superimposed on that produced with the universal constants. The two curves, together with the calculated values of
relationship superimposed on that produced with the universal constants. The two curves, together with the calculated values of  and
and 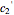 , are presented in the forms of
, are presented in the forms of  versus
versus  (top) and
(top) and  versus
versus  (bottom) whose ranges can also be selected with sliders. Notice that even for the same polymer, the observed glass transition temperature
(bottom) whose ranges can also be selected with sliders. Notice that even for the same polymer, the observed glass transition temperature  can vary dramatically depending on the method of its determination, the test's conditions, and the heating or cooling rate [2, 3].
can vary dramatically depending on the method of its determination, the test's conditions, and the heating or cooling rate [2, 3].
Note that not all combinations of slider-entered values describe a realistic  versus
versus  relationship. When this occurs, all panel output is replaced by the message "unrealistic entries".
relationship. When this occurs, all panel output is replaced by the message "unrealistic entries".
References
[1] M. Peleg, "On the Use of the WLF Model in Polymers and Foods," Critical Reviews in Food Science and Nutrition, 32(1), 1992 pp. 59–66. doi:10.1080/10408399209527580.
[2] R. J. Seyler, ed., Assignment of the Glass Transition, Philadelphia: American Society of Testing Materials, 1994.
[3] E. Donth, The Glass Transition: Relaxation Dynamics in Liquids and Disordered Materials, Berlin: Springer–Verlag, 2001.
Permanent Citation